UH Case Medical Center launches novel clinical trial using stem cells to prevent amputation
University Hospitals Case Medical Center clinical researchers have launched an innovative clinical trial, unique in its design, which will evaluate the ability of a patient's own stem cells to prevent leg amputations in end stage peripheral arterial disease (PAD).
Led by Vik Kashyap, MD, Division Chief, Vascular Surgery at University Hospitals Case Medical Center's Harrington Heart & Vascular Institute and Professor of Surgery at Case Western Reserve University School of Medicine, the clinical trial is designed to improve blood flow in legs with blocked arteries by attempting to treat diseased blood vessels. Peripheral arterial disease (PAD) is a common yet serious disease that occurs when extra cholesterol and fat circulating in the blood collects on the walls of the arteries that supply blood to the limbs.
Due to the location and extent of the blockages in certain individuals, standard treatments such as surgical bypass (insertion of a vein or synthetic graft to redirect blood flow around the blockage) and angioplasty (insertion of a balloon through the artery to open the blockage) will not improve blood flow to the leg, and amputation is the only alternative.
For patients with critical limb ischemia (CLI) revascularization procedures such as surgical bypass or percutaneous angioplasty/stenting are currently the only option to restore perfusion and maintain limb viability.
For CLI patients who are non-candidates for revascularization, amputation is often needed. It is estimated that over 160,000 amputations are performed in the United States each year.
The number of CLI patients who will not be candidates for revascularization continues to rise as the population ages and the incidence of diabetes and other vascular risk factors increase. For CLI patients who are considered unreconstructable, the amputation and mortality rates at six months approach 40% and 20%, respectively. Furthermore, nearly 30% of patients who undergo below-knee amputation will fail rehabilitation and require chronic institutional care or professional assistance at home.
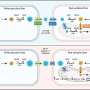
The trial sponsor, Biomet Biologics (Warsaw, IN), recently completed a Phase I study of 30 subjects to evaluate the safety of autologous concentrated bone marrow aspirate for critical limb ischemia. The results of this study were used to advance the company's MarrowStim™ concentration technology into the FDA-approved, pivotal IDE trial described here. Overall, the trial will enroll 152 subjects at up to 20 investigational sites.
"This trial offers an opportunity to save a patient's leg when there are no remaining options to improve blood supply," said Dr. Kashyap. "We are pleased to add this capability at UH and provide hope for patients facing the risk of limb loss."
Subjects will be randomized to receive either the investigational treatment involving the MarrowStim™ P.A.D. Kit (75% chance), or a placebo control involving a sham procedure (25% chance). The trial's primary end point of time to treatment failure, defined as major amputation or death, will be evaluated over a one‐year follow‐up period. Secondary end points, including rest pain, perfusion measurements, quality of life, and safety, will also be evaluated for one year.
More information: Only those patients meeting the pre-defined approved inclusion/exclusion criteria are eligible for this clinical trial. To learn more about this clinical trial and to see the qualifications for participation, visit www.clinicaltrialspotlight.com or call toll-free at 877-788-3972.