Scientists provide insight into the pathology of Sanfilippo A syndrome
Sanfilippo A syndrome or Mucopolysaccharidosis IIIA (MPS-IIIA) is a rare genetic lysosomal storage disease inherited from the parents of the patient. Lysosomes are the body's vehicle for breaking down many of its by-products such as proteins, nucleic acids, carbohydrates, lipids and cellular debris. The spherical vesicles are known to contain 50 different enzymes which are all active around an acidic environment of about pH 5.
Whilst each lysosomal disorder results from different gene mutations that translate into a deficiency in enzyme activity, they all share a common biochemical characteristic, which is when the enzyme sulfamidase is present in too small an amount or is missing completely in the cell. When this occurs, substances usually broken down by the cell as unwanted matter accumulate in the cell, leading to severe problems.
Affected children of the disease show developmental delay, behavioural abnormalities such as hyperactivity, and signs of neurodegeneration such as progressive loss of cognitive and motor functions, cerebral convulsions and spastic quadriplegia.
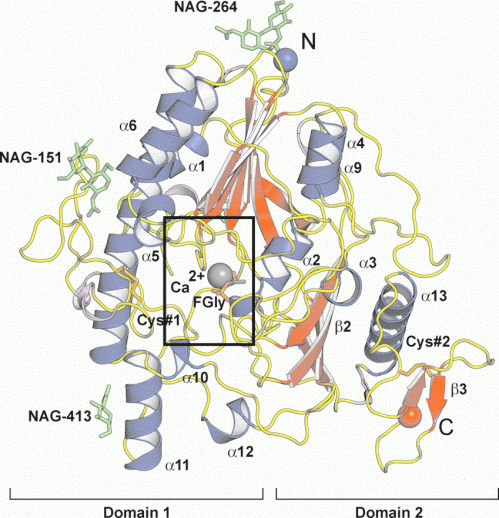
About 80% of the genetic alterations in sulfamidase represent replacement of single amino acids that result in functional inactive enzyme mutants. However the molecular understanding of the effects of these mutations has been confined by a lack of structural data for this enzyme.
A group of scientists from Germany and Spain have been successful in resolving the crystal structure of sulfamidase which provides convincing evidence for the molecular consequences of these amino acid replacements and is fundamental for the development of successful structure-based drug design for this devastating neurodegenerative disorder.
Key features for the successful development of novel therapeutic molecules comprise their specific activity to increase residual enzymatic activity of sulfamidase mutants and their ability to pass the blood brain barrier.
The knowledge of the structural features of sulfamidase will greatly facilitate the discovery of suitable compounds and drugs to assist in managing the disease and its debilitating effects.
More information: Sidhu et al, Acta Cryst. (2014), D70, 1321-1335 DOI: 10.1107/S1399004714002739














