Team examines DNA damage, repair and risk of cancer
(Medical Xpress)—Human cellular DNA endures more than 30,000 daily damages due to everyday, functional activity in and around those cells. Of these damages, about one third are due to oxidative damage which is normally repaired through the work of DNA repair enzymes. Oxidative DNA damage from normal metabolic activity in a cell can lead to permanent and mutable DNA damage if not repaired. Susan Wallace, Ph.D., professor and founding chair of the University of Vermont Department of Microbiology and Molecular Genetics and member of the Vermont Cancer Center, has spent a career looking at the processes involved in, primarily, endogenous DNA damage and repair. Her current work digs deeper and aims to more fully understand how "normal" oxidative stress on cellular DNA and the repair process relates to an increased risk for cancer.
Having spent decades studying oxidative DNA damage and the mechanisms by which DNA protects itself from oxidative stress, Wallace is now taking a transdisciplinary approach to examining the molecular actions and structures involved in DNA repair. By using a variety of techniques – chemical, enzymological, immunochemical, molecular biological, molecular genetic, bioinformatic, x-ray crystallographic and single molecule approaches – and partnering with experts in each of these areas, she hopes to clarify repair processing and the consequences of unrepaired DNA lesions. This unique approach alone is setting a new bar in cancer research, and the implications are critical to determining cancer risk at the molecular level.
Backed by two ongoing National Cancer Institute/National Institutes of Health grants, a history of successful discovery and publications in the process of DNA repair, and numerous cross-disciplinary partnerships, Wallace and her team are working to define the relationship between mutations resulting from normal oxidative stress and predisposition for cancer. Through the use of bioinformatics and crystallographic technologies Wallace has been able to estimate that proteins produced by mutant DNA could be functionally abnormal. Looking at the actions of these mutant proteins has led to understanding of tumor development and assessment of single nucleotide differences in DNA structure which may predispose one to cancer.
In collaboration with Joanne Sweasy, Ph.D., professor of therapeutic radiology and genetics at Yale University and a professor of microbiology and molecular genetics at UVM, Wallace is providing evidence that germline mutations in repair enzymes (SNPS), or mutations in the repair enzymes that are transmitted to offspring, are associated with cancer risk, supporting the idea that cancer can arise due to loss of functional DNA repair enzymes. Wallace and Sweasy are also looking at mutations – called single nucleotide polymorphisms or SNPS – from tumor DNA as a way to determine a personalized approach to cancer treatment.
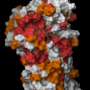
Wallace's collaborations with David Warshaw, Ph.D., professor and chair of molecular physiology and biophysics, have looked at DNA glycosylases, the enzymes that catalyze the first step in base excision repair. By learning more about this mechanism, which repairs DNA damage throughout the cell cycle, they hope to determine how these glycosylases locate damages in a sea of undamaged DNA. Through a continuing partnership with Sylvie Doublié, Ph.D., professor of microbiology and molecular genetics, expert in x-ray crystallography and Pew Scholar, Wallace and Warshaw been able to use X-ray crystallographic analysis to establish the protein structures of glycosylases, which has provided major insight to the function of these enzymes. With this understanding, Wallace and Warshaw have visually determined – in real time – that DNA glycosylases serve as a type of quality assurance agent, inserting themselves into DNA, assessing, repairing if necessary, or, moving on.
Wallace is also working with Marie Wood, M.D., professor of medicine and director of the Vermont Cancer Center's Familial Cancer Program, Jeffrey Bond, Ph.D., professor of microbiology and molecular genetics, and Sweasy, using UVM's Massively Parallel Sequencing Facility. The team is sequencing genetic coding in sibling pairs of DNA from Wood's high-risk cancer registry to look for mutations that confer risk (excluding the more well-known breast cancer-related BRCA genes). This family-based approach will enable Wallace and Wood to identify penetrant, rare genetic variants or multiple mutations in related genetic pathways and relate them to risk of cancer.
Wallace, whose scientific career spans more than 40 years, is excited by this new research and the numerous collaborations that have been sparked across disciplines here at UVM and beyond. This targeted work in DNA repair and identifiable cancer risk has implications for personalized treatment of cancer, early detection, risk mitigation and more. In addition, findings in this area of research could help elucidate potential environmental effects on those individuals already at higher risk for DNA damage—for instance the added risk for certain individuals' exposure to air pollution, or chemicals.
Wallace has ignited a new chapter in science's approach to understanding DNA repair and its relation to establishing risk for cancer. Along with Warshaw, Wood, Doublié, Sweasy, Bond and other partners, as well as countless undergraduate, graduate and post-doctoral student trainees, she is leading the way toward mapping the key markers that will help us predict risk for cancer and other diseases in the future.