Zebrafish model study explains clinical phenotype similarity in megalencephalic leukoencephalopathy patients

Megalencephalic leukoencephalopathy (MLC) is a genetic neurodegenerative disorder affecting the myelin that remains quite unknown. To date, there is not any treatment for patients. This rare disease is caused by mutations in MLC1 and GlialCAM and produces megalencephaly, spasticity and ataxia in humans. A new study of the University of Barcelona, published in the journal Human Molecular Genetics, first describes a phenotype of this human disease through the study of genetically-modified zebrafish models.
The article is signed by Raúl Estévez, Sònia Sirisi and Alejandro Barrallo, from the Department of Physiological Sciences II on the Bellvitge Health Sciences Campus at the University of Barcelona (UB) and members of the Centre for Biomedical Network Research on Rare Diseases (CIBERER); and Virginia Nunes, lecturer of Genetics at UB, researcher at the Bellvitge Biomedical Research Centre (IDIBELL) and member of CIBERER, among other experts.
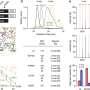
Raúl Estévez explains that "patients with recessive mutations in either MLC1 or GLIALCAM show the same clinical phenotype". "However, it is known that gene GlialCAM regulates the activity of two proteins, MLC1 and the chloride channel ClC-2. Therefore, the absence of the gene GlialCAM should produce a severer phenotype than the lack of MLC1".
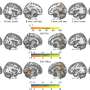
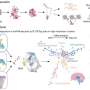
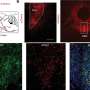
In order to understand this contradiction, the research group led by Raúl Estévez and Alejandro Barrallo compared genetically-modified zebrafish and mice models —in other words, knock-out organisms that, in this case, do not express the gene MLC1— with the brain biopsy from an MLC patient.
Conclusions state that the lack of MLC1 expression causes the delocalization of protein GlialCAM which mimics neuronal activity in all studied models. Genetic modification in zebrafish models is cheap and easy, so authors point out that future research on these animal models will contribute to understand the molecular relationship that exists between the proteins involved in the physiopathology of megalencephalic leukoencephalopathy.
The article is signed by Raúl Estévez, Sònia Sirisi and Alejandro Barrallo, from the Department of Physiological Sciences II on the Bellvitge Health Sciences Campus at the University of Barcelona (UB) and members of the Centre for Biomedical Network Research on Rare Diseases (CIBERER); and Virginia Nunes, lecturer of Genetics at UB, researcher at the Bellvitge Biomedical Research Centre (IDIBELL) and member of CIBERER, among other experts.
Raúl Estévez explains that "patients with recessive mutations in either MLC1 or GLIALCAM show the same clinical phenotype". "However, it is known that gene GlialCAM regulates the activity of two proteins, MLC1 and the chloride channel ClC-2. Therefore, the absence of the gene GlialCAM should produce a severer phenotype than the lack of MLC1".
In order to understand this contradiction, the research group led by Raúl Estévez and Alejandro Barrallo compared genetically-modified zebrafish and mice models —in other words, knock-out organisms that, in this case, do not express the gene MLC1— with the brain biopsy from an MLC patient.
Conclusions state that the lack of MLC1 expression causes the delocalization of protein GlialCAM which mimics neuronal activity in all studied models. Genetic modification in zebrafish models is cheap and easy, so authors point out that future research on these animal models will contribute to understand the molecular relationship that exists between the proteins involved in the physiopathology of megalencephalic leukoencephalopathy.
More information: "Megalencephalic Leukoencephalopathy with subcortical Cysts protein 1 regulates glial surface localization of GLIALCAM from fish to humans." Sirisi S, Folgueira M, López-Hernández T, Minieri L, Pérez-Rius C, Gaitán-Peñas H, Zang J, Martínez A, Capdevila-Nortes X, de la Villa P, Roy U, Alia , Neuhauss S5, Ferroni S, Nunes V, Estévez R, Barrallo-Gimeno A. Human Molecular Genetics. 2014 May 12. pii: ddu231. www.ncbi.nlm.nih.gov/pubmed/24824219