Genentech Alzheimer's drug misses goals in studies (Update)
An experimental drug from the biotech company Genentech failed to slow mental decline in mid-stage studies on more than 500 people with mild to moderate Alzheimer's disease, but showed some promise in the least impaired participants who received a higher dose.
Pneumonia and deaths were more common among those receiving the drug, but researchers downplayed that. Study leader Dr. Jeffrey Cummings of the Cleveland Clinic said none of the deaths seemed due to the drug, and pneumonia occurred at a rate to be expected in older people.
"We're very encouraged" by the hint of benefit for patients with milder dementia and will talk with regulators about next steps for the drug, crenezumab, said a Genentech scientist, Dr. Carole Ho. The results fit with other evidence suggesting that treating earlier in the course of the disease is better, she said.
Results were revealed Wednesday at the Alzheimer's Association International Conference in Copenhagen. Genentech and its corporate parent, Switzerland-based Roche Holding AG, paid for the study and Cummings is a paid adviser to Genentech.
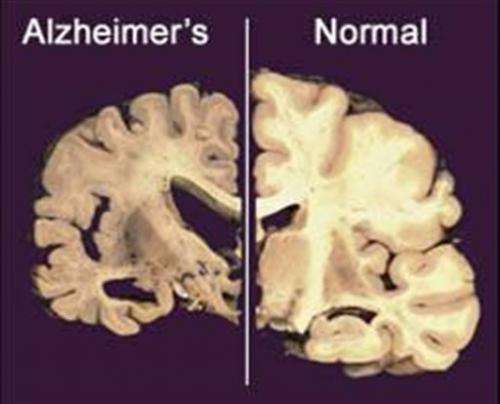
They are the latest mixed bag on treatments aimed at clearing away the sticky amyloid plaques clogging Alzheimer's patients' brains. About 35 million people worldwide have Alzheimer's, the most common form of dementia. There is no cure and current treatments only temporarily ease symptoms.
Last year, an Eli Lilly & Co. medicine, solanezumab, that also sought to clear away amyloid missed main goals in two studies, but combined results suggested it might help people with milder disease. It's in further study now. Before that, bapineuzumab, a similar drug being developed by Pfizer Inc. and Johnson & Johnson, showed promise in mid-stage testing but flopped in larger, more definitive trials.
The Genentech drug has been closely watched because it targets amyloid more broadly than the other drugs do, and the California-based company has a long track record of success with many biological medicines against cancer.
Mid-stage studies aim to give some idea of safety and whether the drug is effective enough to advance to larger, more definitive studies aimed at winning market approval.
In one study, 431 patients ages 50 to 80 with mild to moderate Alzheimer's were given crenezumab or dummy drug as shots every two weeks, or as a higher dose in infusions every four weeks for 17 months. No significant difference was seen among the groups on two widely used measures of thinking and functioning skills.
However, the 70 most mildly impaired participants who received the higher dose declined 35 percent less on the cognitive measure than the 33 mildly impaired people given dummy infusions. The difference was about 3.5 points on the roughly 70-point scale—"equivalent to six or nine months" of delay in decline, Cummings said.
This result isn't definitive, though, and can only be considered a signal worth exploring in future research because it didn't involve the whole group tested. And even in this mildly impaired group, the drug did not improve the second measure, ability to function in daily life.
In the second study, 73 people who showed amyloid plaques on brain imaging also were given crenezumab or dummy shots or infusions. The main outcome—levels of amyloid seen on brain imaging after treatment—will be presented at a medical conference in November. Results on cognitive function seem to mirror those in the larger study, Cummings said.
Five people given crenezumab died—one from sudden death, two from respiratory failure, one from pneumonia and one from worsening Alzheimer's.
"We believe that the safety profile is acceptable," because deaths do not seem related to the drug, Genentech's Ho said.
In a statement Wednesday, the Alzheimer's Association noted that crenezumab was being tested in another study aimed at preventing the disease, and said the new results give hope it will be more successful in that setting.
More information: National Institute on Aging: www.nia.nih.gov/Alzheimers
Patient, family info: www.alzheimers.gov/
Alzheimer's Association: www.alz.org
© 2014 The Associated Press. All rights reserved.