Research partnership results in FDA-approved obstetric medical device

A partnership between researchers at UALR and the University of Arkansas for Medical Sciences has resulted in an inexpensive, disposable external medical device that monitors contractions in pregnant women.
James D. Wilson, assistant director of research at the UALR Graduate Institute of Technology; Dr. Curtis Lowery, chairman of the UAMS College of Medicine's Department of Obstetrics and Gynecology; and Hari Eswaran, Ph.D., a professor in the same department, created and developed the device, which was recently approved by the Food and Drug Administration.
The tocodynamometer, marketed as Koala Toco, was manufactured and marketed by Murray, Utah-based Clinical Innovations, and works with existing equipment such as fetal heart rate monitors.
Work on developing the device started about four years ago, according to Wilson, who said older tocodynamometers are much more expensive and must be cleaned following each use.
Tocodynamometers are electronic devices for monitoring and recording uterine contractions during labor. They are applied to the lower part of the uterus using a belt.
The new FDA-approved device is a small plastic disk that rests on the abdomen of a pregnant woman. When the uterus contracts, it pushes against the intrauterine wall and makes internal pressure rise.
"That pushes the air inside, which produces a signal. The signal is transmitted through an attached cable that also is plugged into a fetal heart rate monitor," said Wilson.
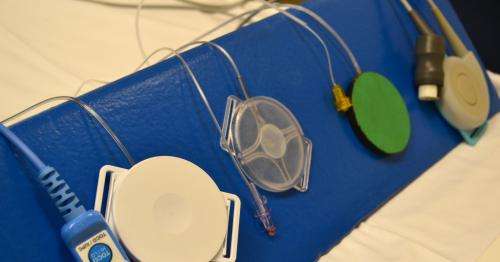
The older, electronic tocodynamometers are heavier and must be continually cleaned because perspiration, other bodily fluids, and medical gels come into contact with it, according to the researchers.
"Because Koala Toco costs about $15, the nurse doesn't have to spend time later cleaning and sterilizing the device. Ours can be tossed out," Wilson added.
Wilson most recently collaborated with Dr. William Culp, a professor of radiology, surgery, and neurology and vice chairman of research at UAMS, to develop the ClotBust ER, which fits on the head like a halo to quickly bust clots that cause stroke.