Optogenetics without the genetics: Nanoparticles enable stimulation of normal, non-genetically modified neurons
Light can be used to activate normal, non-genetically modified neurons through the use of targeted gold nanoparticles, report scientists from the University of Chicago and the University of Illinois at Chicago. The new technique, described in the journal Neuron on March 12, represents a significant technological advance with potential advantages over current optogenetic methods, including possible use in the development of therapeutics toward diseases such as macular degeneration.
"This is effectively optogenetics without genetics," said study senior author Francisco Bezanilla, PhD, Lillian Eichelberger Cannon Professor of biochemistry and molecular biology at the University of Chicago. "Many optogenetic experimental designs can now be applied to completely normal tissues or animals, greatly extending the scope of these research tools and possibly allowing for new therapies involving neuronal photostimulation."
Optogenetics, the use of light to control neural activity, is a powerful technique that has seen widespread use in neuroscience research. It involves genetically engineered neurons that express a light-responsive protein originally discovered in algae. This allows scientists to stimulate individual neurons as well as neural networks with precise flashes of light. However, since optogenetics is reliant on genetic modification, its use is primarily limited to relatively few model organisms.
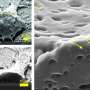
Bezanilla and his colleagues have previously shown that normal, non-genetically modified neurons can be activated by heat generated from pulses of infrared light. But this method lacked specificity and can damage cells. To improve the technique, they focused on gold nanoparticles - spheres only 20 nanometers in diameter, more than 300 times smaller than a human blood cell.
When stimulated with visible light, spherical gold nanoparticles absorb and convert light energy into heat. This heating effect, which is most efficient using green light, can activate unmodified neurons. However, nanoparticles must be extremely close to a cell to produce any effect. Since the nanoparticles diffuse quickly, or get washed away in a neuron's immediate environment, their efficacy is short-lived.
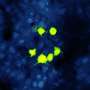
To get nanoparticles to stick, Bezanilla and his team coupled them to a synthetic molecule based on Ts1, a scorpion neurotoxin, which binds to sodium channels without blocking them. Neurons treated with Ts1-coupled nanoparticles in culture were readily activated by light. Untreated neurons were non-responsive. Importantly, treated neurons could still be stimulated even after being continuously washed for 30 minutes, indicating that the nanoparticles were tightly bound to the cell surface. This also minimized potentially harmful elevated temperatures, as excess nanoparticles were washed away.
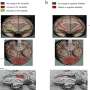
Neurons treated with Ts1-coupled nanoparticles could be stimulated repeatedly with no evidence of cell damage. Some individual neurons, targeted with millisecond pulses of light, produced more than 3,000 action potentials over the span of thirty minutes, with no reduction in efficacy. In addition to cultured cells, Ts1-coupled nanoparticles were tested on complex brain tissue using thin slices of mouse hippocampus. In these experiments, the researchers were able to activate groups of neurons and then observe the resulting patterns of neural activity.
"The technique is easy to implement and elicits neuronal activity using light pulses. Therefore, stimulating electrodes are not required," Bezanilla said. "Furthermore, with differently-shaped nanoparticles it can work in near-infrared as well as in visible wavelengths, which has many practical advantages in living animals. Thus far, most optogenetic tools have been limited to visible wavelengths."
While Ts1 was effective, it did not allow the stimulation of non-Ts1-responsive neuronal populations. To develop a more general strategy of cell targeting, the researchers coupled nanoparticles to antibodies that target other highly expressed proteins in neurons. They chose two antibodies that bind the ion channels TRPV1 and P2X3. Similar to Ts1, neurons treated with these antibody-coupled nanoparticles were activated by light even after continuous washing.
That nanoparticles can be coupled to different antibodies and retain efficacy suggests flexibility for future applications, including human therapeutic development. In retinal diseases such as age-related macular degeneration, for example, photoreceptor cells that absorb light signals are damaged or dead. However, the retinal nerve cells that carry visual information to the brain often remain intact and healthy. Nanoparticles targeted to these cells could potentially absorb light and directly stimulate the neurons, bypassing defective photoreceptors, according to the authors.
"While much additional research must be done to determine the feasibility of this nanoparticle approach as a vision restoration therapy, our results encourage further effort aimed at achieving this critical clinical objective," said study co-author David Pepperberg, PhD, Searls-Schenk Professor of ophthalmology and visual sciences at UIC.
Although no harmful effects were observed, the team notes that toxicity is a possibility. However, many live-animal tests and human clinical trials have already been completed using formulations of gold nanoparticles without serious side effects. The researchers are now testing the efficacy of the technique in animal models to verify its potential for therapeutic use.
More information: "Photosensitivity of Neurons Enabled by Cell-Targeted Gold Nanoparticles," Neuron, 2015.