Researchers identify a new trigger of cellular self-destruction
Researchers have identified a bacterial protein that triggers a self-inflicted cell death pathway in immune system cells and could lead to a better understanding of an important cellular structure.
The protein initiates a cascade of events that leads the lysosome, a cellular structure filled with enzymes that break down and digest cellular material, to open holes in its membrane and release enzymes that destroy the cell, said Zhao-Qing Luo, an associate professor of biological sciences at Purdue University who led the research.
"Immune cells have various ways to detect and defend against the presence of pathogens or danger, and cellular death induced by molecules from the pathogens is a common way to fight infection because it cuts off the stream of nutrients and supplies to the pathogen," he said. "However, our understanding of lysosomal cell death is at an early stage despite the fact that the lysosomes play important roles in the development of many diseases. This protein could be a powerful tool to study that process."
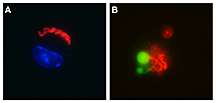
The role of the protein, RpsL, was identified in experiments using Legionella pneumophila, the bacteria that causes Legionnaires' disease. The team found that macrophages, a type of white blood cell that recognizes and engulfs foreign entities in the bloodstream, detected the protein when it was released into the cells by the bacetria and initiated cellular suicide to contain the infection. The findings of the study are detailed in a recently published paper in the journal PLOS Pathogens. The study used macrophages from mice, but RpsL is highly conserved among diverse bacteria and appears to be a universal signature recognized by macrophages, Luo said.
The lysosome used to be considered the trash can of the cell, where cellular debris and molecules that had served their roles were sent to be broken down, but it is emerging as an important structure, Luo said.
"It turns out that the lysosome - once considered a waste bin - is actually a treasure box," Luo said. "Abnormal function of the lysosomes has been seen in diseases including cancer and Alzheimer's disease. It is clear we do not know all of the roles it plays and processes in which it is involved, and it could be a valuable target for therapeutics to improve human health."
The lysosome is a sac filled with eznymes that work like knives to slice molecules into smaller subunits that can be reused in the cell. It contains more than 60 different enzymes and is able to selectively release enzymes to accomplish different tasks. How it creates small holes in its membrane and controls which enzymes are released under different conditions is not known, Luo said.
"No one knows how the selective release of the enzymes within the lysosome occurs and no one had been able to induce the process," Luo said. "Now we can. We know that in the presence of this protein, holes open in the lysosome and enzymes are released. This is a critical ability needed to study lysosome function."
The team next plans to try to find the macrophage receptor to this protein and uncover the full cascade of steps and mechanisms involved in the process. The team also will investigate whether the protein triggers the same response in human macrophage cells.
More information: "Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection." PLoS Pathog 11(3): e1004704. DOI: 10.1371/journal.ppat.1004704