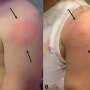
Drug regulations tied to fewer prescriptions of effective gout drug
Well intentioned, but costly and potentially problematic. That's how researchers describe the end result of a decision by the Food and Drug Administration (FDA) to regulate colchicine, a drug used to treat gout, among other ailments. Fewer patients are actually now taking it, and it has come at a cost to their wallets, says study leader Aaron Kesselheim of Brigham and Women's Hospital and the Harvard Medical School in the US. The findings appear in the Journal of General Internal Medicine.
Colchicine had been sold at low cost for many years in the US. It is widely used to treat gout, and is the primary treatment for a rare inflammatory disease called familial Mediterranean fever. Its availability on the US market predated the FDA's regulatory authority to ensure the safety and effective use of prescription drugs, and numerous manufacturers made the drug, selling it for about $0.09 per pill.
In 2007, with the FDA's encouragement, one of these manufacturers conducted a small clinical trial demonstrating the safety and efficacy of a short course of the drug in managing acute flares of gout. The FDA approved this manufacturer's version, granting sole market rights and ordering all unapproved versions off the market by January 2011. Exclusive rights for at least seven years were also granted for its use to treat familial Mediterranean fever, although no new studies were done on its effectiveness to treat this disease. The manufacturer of the new version, called Colcrys, sold the drug for about $5.00 per pill.
To gauge the impact of these market changes, Kesselheim and his colleagues conducted a retrospective cohort study of close to 217,000 commercially insured patients. Between 2009 and 2012, these patients were all newly diagnosed with either gout or familial Mediterranean fever.
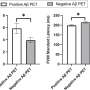
They found that the odds of a familial Mediterranean fever patient being prescribed colchicine within 30 days of receiving such a diagnosis dropped by 7.6 percent per month after the regulations were put in place, while the odds for gout sufferers dropped by 0.5 percent per month. They also found that average patients' monthly total prescription bills rose from $418 to $651.
One of the FDA's justifications for Colcrys' approval was new warnings in Colcrys' label about the potentially lethal risks of co-prescribing colchicine with the antibiotic clarithromycin. But Kesselheim and his colleagues found no change in the rates of co-prescriptions of these two drugs, or co-prescription of colchicine with the transplant rejection medication cyclosporine, another potentially deadly combination.
Kesselheim and his team say that although the FDA's attempts to strengthen its regulatory oversight related to unapproved drugs are laudable, it has come at a price to those who need colchicine. "The way this case was handled has led to a potentially useful drug, colchicine, being prescribed to fewer patients, while there have also been substantial cost increases for those who do use it and no evidence of reductions in unsafe co-prescriptions," says Kesselheim, in summarizing the findings.