Boston Children's and Edwards Lifesciences launch pulmonary valve replacement trial
Surgeons in the Heart Center at Boston Children's Hospital have partnered with Edwards Lifesciences to launch a clinical study of a new prosthetic heart valve for patients born with a congenital heart defect. Called the COMMENCE - Pulmonary Valve Trial, the study will include up to 10 pediatric heart centers from around the U.S.; Boston Children's is the trial's lead site.
Approximately 1 in 100 children are born with a congenital heart defect in the U.S. every year. Of those that require surgical intervention, "valve abnormalities are one of the most common," says Christopher Baird, MD, director of the Congenital Heart Valve Program at the Boston Children's Hospital Heart Center. "They can exist in isolation but also can coincide with other congenital or acquired defects."
More than 100 patients receive prosthetic valve replacements at Boston Children's Hospital every year. However, no prosthetic pulmonary replacement valve has yet received approval from the Food and Drug Administration for use in children or adults. The pulmonary valve replacements patients currently receive are therefore technically conducted on an off-label basis. The FDA approved COMMENCE - Pulmonary Valve Trial will provide experience and data to support an ultimate approval of this application by Edwards Lifesciences.
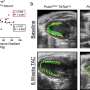
Boston Children's is serving as the primary site for this prospective study, which is expected to enroll approximately 100 patients, ages five and up. Each patient will undergo a procedure in which his or her pulmonary valve—which releases blood from the heart to be oxygenated by the lungs—will be replaced with an investigational prosthetic valve developed by Edwards. The study device is based on Edwards' PERIMOUNT valve platform and incorporates the novel RESILIA tissue, which is designed for extended durability. Patients will be monitored for five years following their procedure.
Baird and his colleagues performed the first successful patient implant in December 2015.
"We enrolled two more patients soon after," said Michele Borisuk, NP, a nurse practitioner in the Congenital Heart Valve Program. "All three are doing well and were able to return home approximately five days after their operations."
"We are very excited about initiating this new trial, as there has been a need for pediatric focused valve trials for many years, " Baird said. "People with congenital heart disease must endure a lifetime of heart treatments, and we are hopeful that our study will provide insight into new options for helping these patients."
Pedro Del Nido, MD, National Co-Principle Investigator and chief of cardiac surgery at Boston Children's and the trial's other national co-principal investigator, added that "further studies in other valve positions are important for this patient community, and we are encouraged that FDA is supportive of investigating new technologies to help pediatric patients."