UH Seidman Cancer Center first in the world to apply SBRT
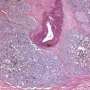
University Hospitals Seidman Cancer Center physicians have started the world's first clinical trial using a new form of stereotactic body radiotherapy (SBRT) to deliver radiation to a specific area of the prostate invaded with cancer - instead of the entire gland. The study aims to determine if treating a targeted cancer region within the prostate in early stage prostate cancer can increase treatment options and reduce the side effects of radiation.
If successful, the trial could have a significant impact on the standard of care for men diagnosed with low or intermediate risk prostate cancer.
The trial's first patient was enrolled and underwent therapy earlier this month without any side effects reported to date. The procedure was performed jointly by his urologist, Lee Ponsky, MD, of the UH Urology Institute and a study co-investigator, and his radiation oncologist Rodney Ellis, MD.
"Our hypothesis is that this method may offer a viable treatment option for men with early stage prostate cancer before their cancer advances, while also minimizing or even eliminating life-changing side effects of treatment," said Dr. Ellis, MD, Vice Chairman, Clinical Affairs, Radiation Oncology, and UH Seidman Cancer Center, a co-investigator on the study. "We want to know if response and cure rates using focal radiotherapy within the prostate can achieve two goals: the first, to successfully eliminate localized malignancies when the disease is in extremely early stages, and second, to reduce the treatment's impact on a patient's quality of life."
According to the National Cancer Institute, approximately 80 percent of prostate cancer is identified in the early stages of development, when the lesions are localized within the prostate. Men diagnosed at this stage have two primary treatment options: "active surveillance," where the patient simply undergoes regular monitoring, or alternatively, whole gland therapy with either surgery or targeted radiation therapy applied to the whole prostate. Due to the prostate's close proximity to the bladder and the rectum, whole prostate radiotherapy, at the levels needed to eradicate malignancies, has a significant risk of causing side effects for men, including rectal bleeding and erectile dysfunction.
For men who have a single lesion amenable to focal therapy, the trial offers a minimally invasive treatment completed in three sessions over a week using SBRT. The trial is designed to validate the patented methodology used in the study to select the target volume for partial prostate treatment. The investigators hope to limit side effects for surrounding tissue.
"Prostate cancer remains the sole malignancy where treatment is always delivered to the whole organ in routine practice," says Mitchell Machtay, MD, Chairman of the Department of Radiation Oncology at UH Case Medical Center and Case Western Reserve University School of Medicine, and principal investigator of the new study. "It is time to investigate new and better options for low-risk prostate cancer patients. Radiology advancements in ultrasound, CT, and MRI enable us to accurately identify, measure, and target malignancies more precisely than ever before."
The study will enroll 12 patients who will be selected using a patented methodology, owned by University Hospitals and developed in part by Dr. Ellis, who is also Associate Professor of Radiation Oncology at the School of Medicine. Philips Medical Systems has licensed this technology and is supporting the study along with Elekta and Invivo.