Researchers explore how Zika infection causes microcephaly
Infection with Zika virus disrupts fetal brain development by interfering with the proliferation of human neural progenitor cells (hNPCs), a type of cell that drives neurodevelopment and proliferates into brain and nervous system cells, according to research presented at the American Society of Human Genetics (ASHG) 2016 Annual Meeting in Vancouver, B.C.
Understanding Zika's mechanisms will illuminate how viral infection leads to birth defects such as microcephaly, a condition marked by an abnormally small head and brain size, and could inform the development of therapies and vaccines, the study authors said.
"We set out to study why Zika causes microcephaly and related viruses like dengue virus don't," said Feiran Zhang, PhD, a postdoctoral researcher at Emory University and presenting author on the research. Dr. Zhang and his colleagues at Emory, Johns Hopkins, and Florida State University focused on the effects of the virus in hNPCs, which are highly susceptible to Zika infection. The hNPCs used in this study were derived from healthy skin cells, Dr. Zhang said.
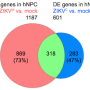
Zika virus was first discovered in Uganda in 1947. Since then, two distinct lineages of the virus have been identified, one African in origin and the other Asian. To compare the effects of each virus on gene expression in these cells, the researchers examined all the messenger RNAs (mRNAs) produced by four groups of hNPCs: cells infected with an Asian strain of Zika virus, cells infected with an African strain of Zika virus, cells infected with a reference strain of dengue virus, and a control group. They found that compared to dengue infection, Zika infection more strongly affects expression of genes involved in DNA replication and repair, processes that are important in brain development.
Interestingly, they found that infection with the Asian strain of Zika virus triggered a stronger innate immune system response than the African strain, including increased expression of the gene TP53. In addition, treatment with p53 inhibitors more strongly inhibited the Asian strain's pathogenicity - its ability to cause harm - than that of the African strain.
"These results suggest that compared to the African strain, the Asian strain's mechanism for causing disease is more dependent on p53," Dr. Zhang explained. He noted that the strain of Zika currently circulating in the Western hemisphere is more similar to the Asian strain than the African one.
In addition to studying mRNA, the researchers also analyzed small RNAs in each group of hNPCs. Both mRNA and small RNAs play important roles in regulating gene expression, Dr. Zhang explained. The researchers observed changes in human small RNAs produced in hNPCs upon Zika infection, as well as in small RNAs directly generated from Zika virus.
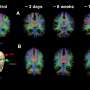
Working in laboratory mice, they injected the most abundant of these small RNAs into the mouse brain to assess the impact of each on brain development, including whether they caused microcephaly. They have identified specific small RNAs from Zika virus that could impact brain development and lead to microcephaly, at least in mice.
"Our results suggest that Zika virus might function as a 'Trojan horse' by 'hijacking' the human cell's machinery," Dr. Zhang said. "Once it infects a human cell, the virus could interact with human enzymes to produce these small RNAs, which could in turn alter brain development and lead to microcephaly."
The researchers are now studying which human mRNAs could be regulated by these small RNAs. They hope their findings will inform the development of preventive and therapeutic approaches.
"It's likely that the mechanisms we found are just some of the many ways in which Zika virus acts," Dr. Zhang said. "We want to identify some important pathways in Zika pathogenesis, which we hope will inspire the experts in treatment and vaccine research," he added.
More information: Dr. Zhang will present his research on Wednesday, October 19, 2016, from 10:15-10:30 a.m., in Room 302 of the Vancouver Convention Centre, West Building.