How protein fragments associated with Alzheimer's could trigger Parkinson's
Alzheimer's and Parkinson's diseases are different neurodegenerative conditions that can sometimes affect the same person, which has led scientists to investigate possible links between the two. Now one team, reporting in the journal ACS Chemical Neuroscience, has identified how amyloid beta, the protein fragment strongly associated with Alzheimer's disease, can induce cellular changes that might lead to Parkinson's.
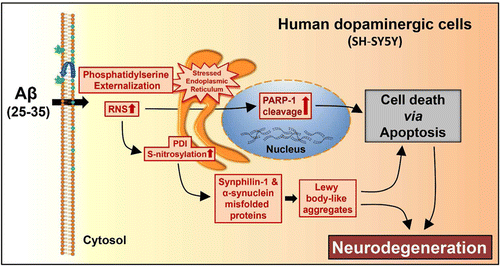
Scientists still don't fully understand what causes these neurodegenerative conditions, but their investigations have revealed some insights. For example, certain molecular changes have emerged as factors in the development of these disorders. One such change is the mutation of an enzyme called protein disulfide isomerase (PDI) that protects neurons. And some research has hinted that biomarkers related to one disease can spur molecular processes leading to others. Mahesh Narayan and colleagues wanted to see how a particular form of amyloid beta might trigger cellular changes that can induce Parkinson's disease.
In their lab, the researchers incubated certain amyloid beta fragments—referred to as Aβ (25-35)—with cells (known as SH-SY5Y) often used in Parkinson's research. This set off in the cells a cascade of molecular changes associated with Parkinson's, including chemical mutations to PDI and the formation of protein clumps known as Lewy bodies. The results could provide an explanation for how someone with Alzheimer's might also develop Parkinson's. The findings also could help researchers discover ways to prevent this from happening.
More information: Parijat Kabiraj et al. An 11-mer Amyloid Beta Peptide Fragment Provokes Chemical Mutations and Parkinsonian Biomarker Aggregation in Dopaminergic Cells: A Novel Road Map for "Transfected" Parkinson's, ACS Chemical Neuroscience (2016). DOI: 10.1021/acschemneuro.6b00159
Abstract
Amyloid beta (Aβ) aggregation is generally associated with Alzheimer's onset. Here, we demonstrate that incubation of dopaminergic SH-SY5Y cells with an Aβ peptide fragment (an 11-mer composed of residues 25–35; Aβ (25–35)) results in elevated intracellular nitrosative stress and induces chemical mutation of protein disulfide isomerase (PDI), an endoplasmic reticulum-resident oxidoreductase chaperone. Furthermore, Aβ (25–35) provokes aggregation of both the minor and major biomarkers of Parkinson's disease, namely, synphilin-1 and α-synuclein, respectively. Importantly, fluorescence studies demonstrate that Aβ (25–35) triggers colocalization of these Parkinsonian biomarkers to form Lewy-body-like aggregates, a key and irreversible milestone in the neurometabolic cascade leading to Parkinson's disease. In addition, fluorescence assays also reveal direct, aggregation-seeding interactions between Aβ (25–35), PDI and α-synuclein, suggesting neuronal pathogenesis occurs via prion-type cross-transfectivity. These data indicate that the introduction of an Alzheimer's-associated biomarker in dopaminergic cells is proliferative, with the percolative effect exercised via dual, independent, Parkinson-pathogenic pathways, one stress-derived and the other prion-like. The results define a novel molecular roadmap for Parkinsonian transfectivity via an Alzheimeric burden and reveal the involvement of PDI in amyloid beta induced Parkinson's.