Experimental drug shows promise for sight-stealing eye condition
An experimental drug may one day make treatment simpler for patients suffering from vision-threatening age-related macular degeneration, researchers say.
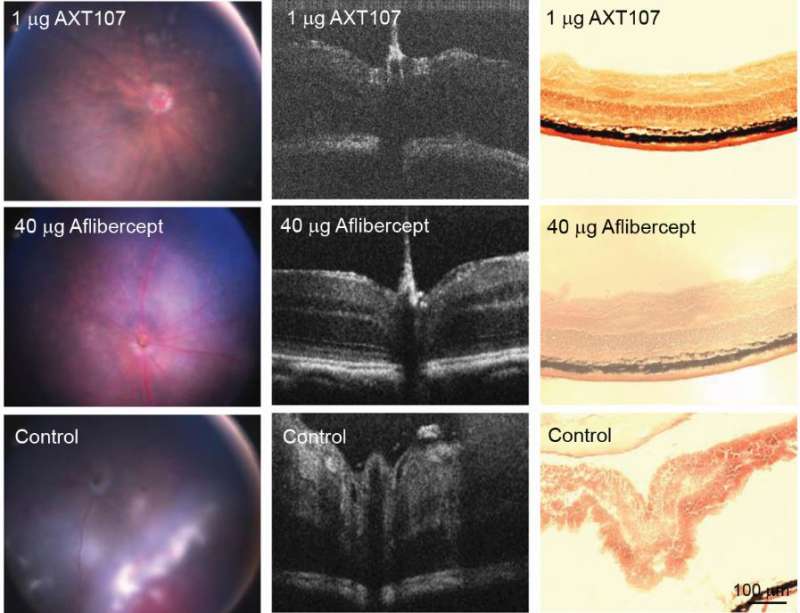
So far, the drug—called AXT107—has been only tested on mice and rabbits, but it requires far fewer injections than current therapy to prevent vision loss. Current treatment requires frequent injections directly into the eye.
"We anticipate injection of AXT107 in humans may have a substantially longer effect than current treatment," said lead researcher Dr. Peter Campochiaro. He's a professor of ophthalmology at Johns Hopkins University in Baltimore.
"Instead of eye injections every four to six weeks, we hope it would be several months between injections," he said.
The drug test in rabbits lasted only two months, but the drug appeared both safe and effective, Campochiaro said.
Researchers hope to start the first human trials later this year. This phase 1 trial would test the safety of the drug in people, but further tests would be required to see how effective it is, Campochiaro said.
Research with animals does not always produce similar results in people.
Age-related macular degeneration is a common eye condition and a leading cause of vision loss among people age 60 and older, according to the U.S. National Institutes of Health.
The condition causes damage to the macula, a small spot near the center of the retina and the part of the eye needed for sharp vision.
In some people, age-related macular degeneration advances so slowly that vision loss doesn't occur for a long time. In others, the disease progresses faster and may lead to a loss of vision in one or both eyes, according to the NIH.
As age-related macular degeneration progresses, a blurred area near the center of vision is a common symptom. Over time, the blurred area may grow larger and blank spots may develop in central vision. Objects also may not appear as bright as they used to.
Age-related macular degeneration by itself doesn't lead to complete blindness, the NIH says. The loss of central vision can, however, interfere with simple activities, such as the ability to see faces, drive, cook, read and write.
One type of this condition is known as "wet" or neovascular age-related macular degeneration. This type develops when new blood vessels form and leak fluid and blood into the eye, causing swelling and damage, according to the NIH.
Treatments for this type of macular degeneration have limited effectiveness and require monthly injections directly into the eye to prevent irreversible vision loss, Campochiaro explained.
But the injections of AXT107 in animals lasted twice as long as the usual drugs, such as aflibercept (Eylea). Aflibercept prevents blood vessel growth by blocking a protein called vascular endothelial growth factor (VEGF).
AXT107 also targets VEGF and three other factors that promote blood vessel growth, Campochiaro said.
Moreover, AXT107 forms a gel within the eye, which allows it to be released over several months, reducing the number of eye injections patients need to control the disease, he said.
"AXT107 may provide a way to get as good or better effects as patients are getting with current treatment, but with fewer visits and injections," Campochiaro said.
The report was published Jan. 18 in the journal Science Translational Medicine.
One eye specialist not involved with the study said the frequency of current treatments can be a problem.
"Patients with age-related macular degeneration are flooding our offices with multiple injections," said Dr. Mark Fromer, an ophthalmologist at Lenox Hill Hospital in New York City.
"We need treatments that last longer, because you have 60- to 80-year-old patients, it's almost impossible for them to come to the office every month. They do it, but it's rough," Fromer said.
The less a patient has to come to the doctor's office, the better the compliance, he said.
"If they can come in five or six times a year, it is much better for patient compliance and may also result in better care," Fromer said. "Anything we can do to reduce patient visits is a plus."
More information: "Tyrosine kinase blocking collagen IV–derived peptide suppresses ocular neovascularization and vascular leakage," Science Translational Medicine, stm.sciencemag.org/lookup/doi/ … scitranslmed.aai8030
Copyright © 2017 HealthDay. All rights reserved.