'Heart-in-a-dish' sheds light on genetics of heart disease

When a patient shows symptoms of cancer, a biopsy is taken. Scientists study the tissue, examining it under a microscope to determine exactly what's going on.
But the same can't be done for heart disease, the leading cause of death among Americans. Not until now.
Dr. J. Travis Hinson, a physician-scientist who joined the faculties of UConn Health and The Jackson Laboratory for Genomic Medicine (JAX) in 2016, is using a novel system he pioneered to study heart tissue.
Hinson engineers heart-like structures with cells containing specific genetic mutations in order to study the genetics of cardiomyopathies, diseases of the heart muscle that can lead to heart failure and, ultimately, death.
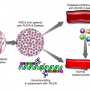
"We basically try to rebuild a little piece of a patient's heart in a dish," says Hinson, who developed the technique during a postdoctoral fellowship at Brigham & Women's Hospital.
He combines cardiac muscle cells with support cells, such as fibroblasts, and other key factors, including extracellular matrix proteins. Although these tiny, three-dimensional structures do not pump blood, they do contract rhythmically, and their beating strength can be studied.
Making a Difference
Hinson is applauded for his ability to move seamlessly between research, clinical practice, and teaching – the three prongs of an academic medical center's mission. He's able to do so, perhaps, because his own career began at the intersection of multiple scientific specialties.
As an undergraduate at the University of Pennsylvania, Hinson interned at DuPont in New Jersey to explore his interests in chemistry and engineering. But he soon realized that his passion for science needed a real-word focus. "I wanted to do science that made a difference in people's health," he says.
The same summer, he volunteered in the emergency department of a local hospital. Impressed by a cardiologist's calm and collected manner in a crisis, and gaining interest in the heart, Hinson changed his career trajectory from engineering to medical school.
Hinson joined the laboratory of Dr. Robert J. Levy, a pediatric cardiologist and researcher at The Children's Hospital of Philadelphia, working to harness gene therapy techniques to make artificial heart valves and other cardiovascular devices more durable. Through this early foray into biomedical research, Hinson deepened his interest in biomedical science and gained an appreciation of the work of a physician-scientist.
While doing research in Dr. Christine Seidman's lab as part of his MD at Harvard Medical School, he chose to lead a project on Björnstad syndrome, a rare, inherited syndrome characterized by hearing loss and twisted, brittle hair. At the time, little was known about the molecular causes of the disorder, although the genetic culprits were thought to reside within a large swath of chromosome 2. Using genetic mapping techniques and DNA sequencing, Hinson homed in on the precise mutations.

In addition to casting light on disease biology, he glimpsed the power of genomic information. "I was fascinated by the potential for understanding new genes that cause human diseases," Hinson says, "and how important that was to society."
Matters of the Heart
Throughout his medical training, Hinson noticed there were some significant stumbling blocks to gathering a deep knowledge of heart disease, particularly cardiomyopathies.
Cardiac muscle has essentially two paths toward dysfunction and ultimate failure. It can either dilate – become abnormally large and distended – or it can thicken. Both routes severely impair how well the heart performs as a pump. These conditions, known as dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM), can stem from pre-existing disorders of the heart, such as a previous heart attack or long-standing hypertension, or from DNA mutations.
Thanks to advances in genomics over the last two decades, more than 40 genes have been identified that underlie cardiomyopathy. But unlike diseases such as cystic fibrosis or sickle cell anemia, where it is fairly common for affected individuals from different families to carry the exact same genetic typo, it is exceedingly rare for unrelated patients with cardiomyopathy to share the same mutation. With such a complex genetic architecture, figuring out how the different genes and gene mutations contribute to heart disease has been an enormous challenge.
Because of this formidable hurdle, drug discovery for the cardiomyopathies has languished. "There really has not been a paradigm-shifting drug developed for heart failure in the last 20 years," says Hinson. Moreover, the few treatments that do exist are primarily aimed at controlling patients' symptoms, not slowing or halting their disease.
Hinson aims to improve this picture. With his "heart-in-a-dish" technique, he and his team are now unraveling the effects of genetic mutations on cardiac biology.
The system harnesses multiple recent advances in both stem cell and genome editing technologies. With these capabilities, Hinson and his colleagues can isolate skin or blood cells directly from cardiomyopathy patients and coax them to form heart muscle cells, making it possible to study the biological effects of patients' own mutations. Moreover, he can correct those mutations, or create additional ones, to further probe how genetic differences influence heart biology.
Part of the allure of Hinson's approach is that it can be readily applied to studying other forms of heart disease. It can also be leveraged for drug discovery, providing a platform to screen and test compounds with therapeutic potential in a wide range of cardiovascular diseases.
In addition to his research lab based at JAX Genomic Medicine, Hinson continues to practice cardiology at UConn Health. He helps run a specialized clinic focused on genetic forms of heart disease, as well as arrhythmias, connective tissue disorders, and other conditions.
"We have an exciting opportunity to provide clinical services in cardiac genetics in the corridor between New York and Boston," he says. That means state-of-the-art genetic testing, including gene panels and genome sequencing, as well as genetic counseling for both patients and family members to help inform disease diagnosis and guide treatment. Although there are only a handful of treatments now available, Hinson believes this clinic will be uniquely poised to take advantage of a new generation of personalized treatments that are precisely tailored to patients' specific gene mutations.
"Travis really is a quintessential physician-scientist," says Dr. Bruce Liang, dean of UConn School of Medicine and director of the Pat and Jim Calhoun Cardiology Center at UConn Health.
"He has a remarkable ability to link basic science with important clinical problems, and his work holds a great deal of promise for developing new treatments for patients with cardiomyopathy. I wish there were two or three Travis Hinsons."