Anti-cell death agent a potential treatment for vision loss associated with multiple sclerosis
A new therapeutic agent tested in a mouse model of multiple sclerosis (MS) produced anti-inflammatory activity and prevented loss of cells in the optic nerve, according to a new study by researchers in the Perelman School of Medicine at the University of Pennsylvania, with Pittsburgh-based Noveome Biotherapeutics. The research was conducted in the laboratory of Kenneth Shindler, MD, PhD, an associate professor of Ophthalmology and Neurology, and published in Scientific Reports.
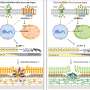
The team demonstrated the therapeutic potential of the agent, called ST266, for treating optic neuritis, inflammation that damages the optic nerve and is a common presenting feature of MS. About half of patients diagnosed with MS experience optic neuritis, which can cause mild to moderate permanent loss of vision, but rarely complete blindness. ST266 is a solution of molecules that stimulate paracrine signaling. This is one way in which cells "talk" to each other: One cell produces a chemical signal that induces changes in nearby cells.
"In this case, the idea is that the many factors in ST266 not only bind to cell receptors and cause changes within the cells they bind to, but those cells then alter their own secretions and provide additional signals to other neighboring cells, thus propagating an effect from a relatively small amount of protein present in the therapy itself," Shindler said. "To the best of our knowledge, this study demonstrates, for the first time, the ability to treat the optic nerve via the intranasal route of administration."
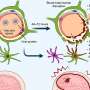
When ST266 was given to the MS mice via their nose, it reached the central nervous system within 30 minutes and was detected at higher concentrations in parts of the eye and optic nerve compared to other areas of the brain. These findings demonstrated that this type of delivery can target tissues of the eye, which is easier, less painful, and less invasive than injecting medication directly into the eye.
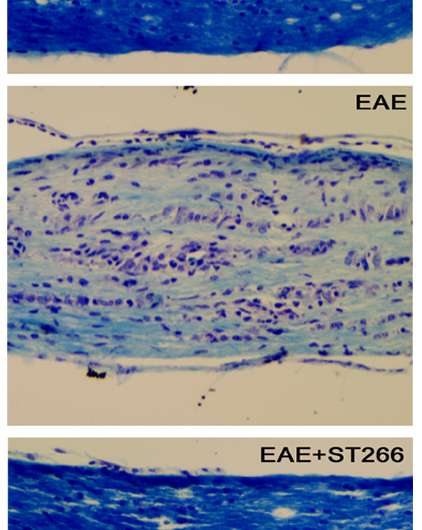
In mice with optic neuritis, the team showed that early treatment with ST266 prevented damage and dysfunction, marked by significantly reduced loss of optic nerve cells, and suppression of inflammatory cell infiltration into the optic nerve. This in turn was associated with limitation of the degree of demyelination caused by MS- related optic neuritis. However, "it's not known if these effects are independent effects of the therapy or interdependent effects," Shindler said.
Treatment of later-stage optic neuritis in the MS mice showed similar results, resulting in improved visual function compared to untreated groups. The data suggest that ST266 helps promote optic neuron survival by potentially activating multiple pathways, including those that prevent cell death.
"These results are particularly important as the preservation of retinal cells is a significant factor when treating optic neuritis," Shindler said. "There is an increased need for combination treatment options that are able to prevent nerve-cell axon loss for patients with optic neuritis."
Currently, the only acute treatment for MS-related optic neuritis is IV steroids, which only hasten whatever amount of visual recovery will occur even without treatment. Steroids do not prevent nerve damage or permanent vision loss. "ST266's ability to preserve vision in the preclinical model and reduce neuronal loss would be a huge advance if it translates to human patients," Shindler said.
The study also has implications beyond MS-related optic problems. "We also showed an effect on cultured neurons, suggesting that effects may translate to other optic nerve diseases, as well as other brain neurodegenerative diseases," Shindler said.
More information: Reas S. Khan et al, Intranasal Delivery of A Novel Amnion Cell Secretome Prevents Neuronal Damage and Preserves Function In A Mouse Multiple Sclerosis Model, Scientific Reports (2017). DOI: 10.1038/srep41768