Tailored intervention in acute and chronic inflammation
Signal molecules called chemokines often work in tandem to recruit specific sets of immune cells to sites of tissue damage. A systematic analysis of their interactions by researchers from Ludwig-Maximilians-Universitaet (LMU) in Munich pinpoints potential targets for new therapies.
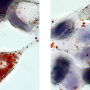
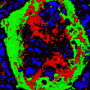
Chemokines are small signal proteins that are secreted by their producer cells, and function as attractants for specific cell types, summoning them to sites in the body where they are needed. Most of these proteins act on cells of the immune system, and recruit them to sites of injury or infection. The cells reach their targets by following the rise in the concentration of the chemokine back to its cellular source in the tissues, a process known as chemotaxis. Hence, chemokines are involved in initiating and regulating inflammation reactions, which are triggered by acute tissue damage or metabolic imbalances. For example, chemokines are intimately involved in the pathogenesis of atherosclerosis, i.e. the localized infiltration with lipid-laden macrophages and deposition of fat-rich debris which can obstruct the flow of blood through major arteries. For these 'plaques' are themselves the product of chronic inflammation reactions. In a paper that has just appeared in the journal Science Translational Medicine, researchers led by LMU's Professor Christian Weber and Dr. Philipp von Hundelshausen now report the results of the first ever systematic survey of direct interactions between individual chemokines and characterized their biological effects.
Different chemokines are capable of binding to each other to form so-called heterodimers, i.e. functional units consisting of two distinct subunits, and such interactions may either potentiate or attenuate their function. This makes heterodimers interesting as drug targets for novel therapies for the treatment of acute and chronic inflammation. "Up to now, however, only one heterodimer had been sufficiently well characterized to allow it to be targeted by synthetic peptides in the context of a therapeutic intervention. In that case, the heterodimer exacerbates the recruitment of monocytes that stimulate atherosclerosis to sites of inflammation in the blood vessel wall," Weber explains.
Weber and his collaborators have now, for the first time, systematically screened all pairwise combinations of the 50 or so known chemokines for their ability to form heterodimers, and identified those interactions that are functionally relevant and potentially targetable for therapeutic purposes. Using an array of analytical methods to probe structure-function relationships and a set of transgenic mouse strains as experimental models, the researchers found that chemokines that are secreted in the course of inflammatory reactions are particularly prone to heterodimerize with each other. Furthermore, the team was able to show that these binding interactions can be classified into two structural types, which are referred to as CC and CXC dimers. "Our results also demonstrate that these two subtypes differ functionally: Heterodimers of the CC class have a more potent chemoattractant effect, and in mouse models they promote acute inflammation of the lung and atherosclerosis. Dimers of the CXC type, on the other hand, repress chemotaxis. So the formation of chemokine heterodimers enables the organism to fine-tune the overall level of chemokine activity," von Hundelshausen says.
"In the course of our study, we were able to demonstrate that specially designed synthetic peptides selectively inhibit the ability of CC heterodimers to promote the development of atherosclerosis and acute inflammation of the lung, or mimic the capacity of CXC heterodimers to inhibit platelet aggregation, thus limiting the risk of thrombosis," Weber says. Appropriately designed peptides could therefore serve as the basis for the creation of new anti-inflammatory and anti-platelet compounds without side effects.
More information: Philipp von Hundelshausen et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation, Science Translational Medicine (2017). DOI: 10.1126/scitranslmed.aah6650