A perturbed skin microbiome can be 'contagious' and promote inflammation, study finds
Even in healthy individuals, the skin plays host to a menagerie of bacteria, fungi and viruses. Growing scientific evidence suggests that this lively community, collectively known as the skin microbiome, serves an important role in healing, allergies, inflammatory responses and protection from infection.
In a new study, researchers at the University of Pennsylvania have shown for the first time that, not only can infection with the Leishmania parasite alter the skin microbiome of affected mice, but this altered microbial community can be passed to uninfected mice that share a cage with the infected animals.
Mice with the perturbed microbiome, or dysbiosis, had heightened inflammatory responses and more severe disease when they were subsequently infected with Leishmania. The findings are published in the journal Cell Host & Microbe.
"To my knowledge, this is the first case where anyone has shown that a pre-existing skin microbiome can influence the outcome of an infection or a disease," said Elizabeth Grice, co-senior author and assistant professor in the departments of Dermatology and Microbiology in Penn's Perelman School of Medicine. "This opens the door to many other avenues of research."
In addition, when the researchers examined samples from human Leishmania patients, they found similar patterns of dysbiosis as in the infected mice, a hint that the findings may extend to people.
"The transmission of dysbiosis in the skin from one animal to another is a key finding," said Phillip Scott, professor of immunology in the Department of Pathobiology in Penn's School of Veterinary Medicine and co-senior author on the study. "And the fact that we saw similar patterns of dysbiosis in humans suggests there could be some very practical implications of our work when it comes to treating people with leishmaniasis."
Grice and Scott collaborated with researchers from Penn Medicine and Penn Vet, including lead author Ciara Gimblet, a Ph.D. student in Scott's lab, and colleagues from Brazil's Oswaldo Cruz Foundation.
Cutaneous leishmaniasis is a tropical disease caused by a parasite and transmitted by the bite of a sand fly. The disease results in sores on the skin, which can sometimes become severe and disfiguring. There is no vaccine for the disease and the limited drugs available often fail to provide a complete cure.
Curious about the influence of the skin microbiome on the disease, the Penn-led team swabbed the skin of 44 Leishmania patients, analyzing the microbiota not only of their lesions but also the area around them and a portion of skin on the opposite side of the bodies as the lesion. They noticed that the lesion samples contained less bacterial diversity than the samples of other skin sites. But not all of them were the same; they found three distinct community types: one dominated by Staphylococcus, one by Streptococcus and one that was mixed.
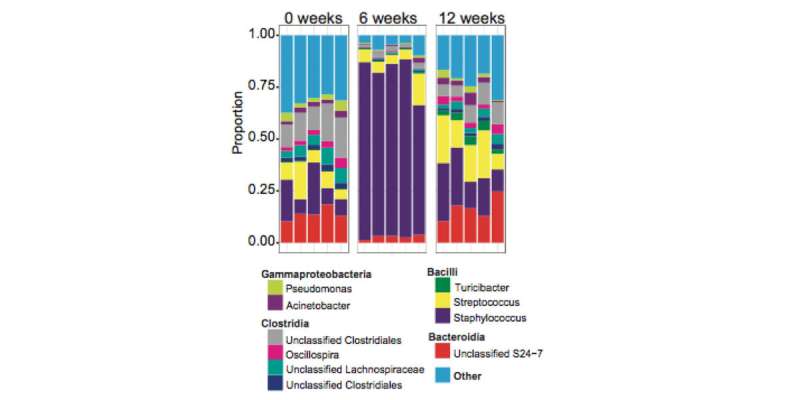
To get a clearer picture of how these microbiome shifts were connected to the disease, the researchers turned to a mouse model of Leishmania infection. Mirroring the findings in humans, the team found that infection with the Leishmania parasite induced a change in the skin microbiota in mice. They also found an association between the microbiota community type and disease severity. In mice that eventually resolved their infections, Staphylococcus dominated in the lesions, while Streptococcus was the dominant species in lesions on mice with a persistent, severe form of the disease.
A major discovery was that these shifts in microbiota were transmissible not only to other parts of the same mouse but to cage mates. When they kept mice infected with Leishmania in the same cage as uninfected mice for six weeks, the uninfected mice acquired a perturbed skin microbiome "profile" that resembled the infected mice.
The researchers hope to see whether the sharing of perturbed microbiota happens not just in mouse cages but also in households.
"I think an important next step will be to see if this sharing of microbiota occurs in people, and whether that could be a factor in affecting the severity of infections in humans," Grice said.
A final question was to determine whether this naturally transmitted dysbiosis would predispose the uninfected animals' response to an enhanced inflammatory response. And indeed, when infected with Leishmania, these mice had more severe inflammation and skin ulcers than mice with unperturbed skin microbiota. In a more general assay, the researchers used a contact hypersensitivity assay, which uses a skin irritant to elicit an immune response, on the mice that had been housed with Leishmania-infected mice. These dysbiotic mice, too, had a heightened inflammatory response.
To follow up on their findings, the researchers hope to examine whether sharing of a dysbiosis occurs in other infections and whether the resulting alteration in skin microbiota affect processes such as wound healing.
In addition, the Penn researchers will be working with their colleagues in Brazil to further examine the connections between the microbiome and leishmaniasis. Specifically, they hope to determine whether there is a connection between the type of skin microbiome present in Leishmania lesions and the severity of disease, or the responsiveness to treatment.
If true, "this may make us rethink the role of antibiotics in treating leishmaniasis," Scott said.
Though previous studies are mixed about the effectiveness of antibiotics in alleviating the disease, additional information about the microbes that exacerbate inflammation could lead to more tailored therapies to tame skin lesions.
More information: Ciara Gimblet et al, Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation, Cell Host & Microbe (2017). DOI: 10.1016/j.chom.2017.06.006