New antibodies target protein structures common to several neurological diseases
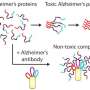
A new kind of antibody targets a feature shared by proteins thought to cause the most damage in Alzheimer's disease, Parkinson's disease, and related conditions, creating potential for a unified treatment approach.
This is the finding of a study led by researchers from NYU School of Medicine and published online August 29 in Scientific Reports.
The new study is based on decades of work arguing that the contribution to disease of key proteins - amyloid beta and tau in Alzheimer's, alpha-synuclein in Parkinson's, and prion proteins in conditions like mad cow disease - is driven by certain, toxic forms dominated by a common structure: bundles of "beta sheets" in clumped proteins.
In tissues from autopsied patients with these diseases and in live mice, experiments demonstrated how the study antibodies target and remove only these toxic forms, say the authors, and without triggering the immune toxicity that has frustrated treatment development efforts to date.
"In an atmosphere where countless treatments have failed in clinical trials over the last 15 years, the fact that our approach continues to be effective in rigorous tests should be of interest to our peers and the industry, even if it runs contrary to conventional thinking," says corresponding author Fernando Goni, PhD, research associate professor in the Department of Neurology at NYU School of Medicine.
"While we still have a number of milestones to reach before this work is ready for clinical testing, our results suggest that these antibodies may halt key pathological mechanisms across several neurological diseases and regardless of disease stage," says corresponding author Thomas Wisniewski, MD, director of the Center for Cognitive Neurology, Silberstein Alzheimer's Institute, and the NYU Alzheimer's Disease Center.
New Approach
The study focuses on proteins that form important structures in the brain. The instant they form as chains of amino acids, proteins fold into complex shapes needed to do their jobs. Unfortunately, proteins can also "misfold" for countless reasons (genetic abnormalities, toxins, age-associated cell processes, inflammation, etc.) that eventually cause the diseases addressed by the current study. Cells and tissues die as misshapen proteins stop working and build up, but the field has struggled to pinpoint which of these shifting forms to target as the key drivers of disease.
Many research efforts, including the current study, seek to design antibodies, which are immune proteins that can attach to and remove a disease-related target molecule. Past and ongoing attempts have used antibodies that target the initial, short chains of amino acids that serve as basic, repeating structural units (monomers) of each misfolded protein. Still others targeted end-stage fibrils, each made of thousands of monomers, which accumulate in plaques and tangles that tissues cannot eliminate. Neither approach has yielded an effective therapy.
In that light, Goni, Wisniewski and colleagues designed their antibodies to target instead the "oligomers" formed as several misfolded monomers associate and acquire the "beta-sheet" shape, but not yet large enough to fibrilize. These intermediate forms may be uniquely toxic, say many in the field, because, unlike fibrils, they can dissolve and move in and out of cells, and from one cell to another. This mobility may explain the "prion-like" progression seen in misfolding diseases where abnormal proteins cause normal ones to misfold in a domino effect that damages nerve cells and their connections in the brain.
Importantly, growing toxic oligomers of amyloid beta, tau, alpha synuclein, and prion protein become increasingly dominated by twisted strands of amino acids, the beta sheet spatial arrangements that let the strands stack up.
To design new kinds of antibodies, the research team zeroed in on a small 13-amino-acid peptide, derived from the extremely rare genetic disease called British amyloidosis, but not present in the rest of the human population. They converted it into large, stable oligomer with more than 90 percent "beta-sheet" structure (the p13Bri immunogen) that could now be "seen" by the mammalian immune system. It also triggered a specific antibody response that solved problems encountered with standard approaches. By immunizing mice with p13Bri at high doses, they forced the production of extremely rare antibodies against beta sheets in toxic oligomers.
The researchers say that their rare antibodies, activated by protein fragments seen only in a rare disease, have almost zero chance of triggering unwanted immune responses to normal proteins with similar sequences (autotoxicity), the downfall of many previous attempts. Finally, the team screened their lead antibodies against tissues taken from the brains of human patients with Alzheimer's, Parkinson's and prion diseases. Only the six monoclonal antibodies that reacted to toxic oligomers from at least two misfolded proteins from two diseases were selected for further study.
"This publication details the first system for making antibodies that truly target only toxic oligomers of misfolded proteins dominated by beta sheets across several diseases, and without regard to the amino-acid makeup of each misfolded protein's monomer," says Goni.