Druglike molecules produced by gut bacteria can affect gut, immune health

Stanford researchers found that manipulating the gut microbe Clostridium sporogenes changed levels of molecules in the bloodstreams of mice and, in turn, affected their health.
Here's some food for thought: When you lick your Thanksgiving plate clean this week, you're not just feeding yourself; you're also providing meals to the trillions of microbes that live in your gut.
And if your dinner includes turkey, a notoriously rich source of the amino acid tryptophan, the gut bacterium Clostridium sporogenes will have the job of breaking down that tryptophan. Then the molecules that are produced by the microbe will flow into your bloodstream in the same way a prescription drug might, interacting with your immune system and changing the biology of the intestines.
Stanford University School of Medicine researchers have used mice to demonstrate how gut bugs could be bioengineered to produce possibly therapeutic changes in the body.
A paper describing their efforts was published online Nov. 22 in Nature. Justin Sonnenburg, PhD, associate professor of microbiology and immunology, and Michael Fischbach, PhD, associate professor of bioengineering, share senior authorship. The lead author is Dylan Dodd, MD, PhD, instructor in pathology.
When the researchers blocked the ability of C. sporogenes to break down tryptophan in mice, levels of certain molecules in their bloodstreams changed. Moreover, the researchers saw physiological changes to the mice's immune systems and intestines.
"This is a vivid example of not only how the microbiome is affecting things all over your body, but of how we can leverage that to improve health," said Sonnenburg, using a term for the collection of microbes living on or inside an animal, or in a particular part.
Improving health from the inside
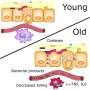
Over the past 15 years, researchers have shown that the composition of a person's gut microbiome can alter their risk for all sorts of health problems, from diabetes and heart disease to allergies and depression. One reason these tiny microbes have such an outsized effect: They can produce molecules known as metabolites that enter the bloodstream and circulate throughout the body. Pinning down exactly which molecules are produced by which bacteria, however, and how to alter their levels to change health, has been challenging.
Previous studies have shown that just a few bacteria, including C. sporogenes, can break down tryptophan and produce the metabolite known as indolepropionic acid. Studies have also hinted that IPA helps fortify the intestinal wall, letting fewer molecules leak through.
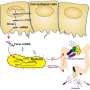
In the new work, the researchers first detailed exactly how C. sporogenes produces IPA from tryptophan. They identified a handful of other compounds also produced in the process—12 metabolites in total, nine of which can accumulate in the blood and three of which are produced only by bacteria. Then, the researchers pinpointed for the first time the genes that C. sporogenes requires for the breakdown of tryptophan and metabolism of the resulting molecules. A gene called fldC, they showed, is required for the production of IPA.
Next, the team gave germ-free mice either wild-type C. sporogenes—with the ability to produce IPA—or a version of the bacteria that lacked fldC. In mice that received the wild-type bacteria, levels of IPA in the bloodstream were around 80 micromolar; in mice that received the engineered version of the bacteria, IPA was undetectable.
Finally, they looked at how altering the levels of IPA affected the mice. Mice with undetectable IPA, they found, had higher levels of immune cells, including neutrophils, classical monocytes and memory T cells. This suggested activation of two branches of the immune system—the innate and adaptive immune system. In addition, the mice with the engineered version of C. sporogenes had more permeable intestines, a defect which is often seen in gut diseases, including inflammatory bowel disease.
Targeting microbes
If the results hold true in humans, said Sonnenburg, it could point toward a new paradigm for treating some diseases: rather than give a compound, such as IPA, physicians may one day be able to tweak levels of bacteria to affect levels of metabolites. For instance, it might be possible to treat inflammatory bowel disease by boosting levels of C. sporogenes and ensuring patients eat enough tryptophan.
"This gives us a specific example of how we can target individual microbes and pathways in the gut to change a person's health," Dodd said. "And this is just one example of hundreds or thousands that are likely out there."
The group next plans to study C. sporogenes and IPA levels in mice with more complex gut microbiomes—rather than germ-free mice—and begin tracking down other metabolites produced by the gut microbes that may have health effects.
"While providing a stunning example of how a single gut microbe, and a single gene within that microbe, can impact host health, IPA is just the tip of the iceberg," said Fischbach, "The possibility to positively impact human health through microbiome-produced chemicals is tremendous, and we are poised to take big strides and make this a reality."
Other Stanford authors are Matthew Spitzer, PhD, a former graduate student; graduate students William Van Treuren and Bryan Merrill; postdoctoral scholar Andrew Hryckowian, PhD; life science researcher Steven Higginbottom, PhD; Gary Nolan, PhD, professor of microbiology and immunology; adjunct faculty member Anthony Le; and Tina Cowan, PhD, professor of pathology.
More information: A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites, Nature (2017). nature.com/articles/doi:10.1038/nature24661