Why certain cancer therapeutics may bind—or fail to bind—to mutant proteins
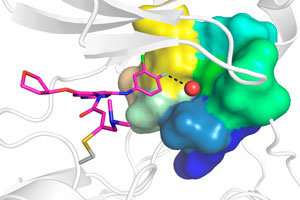
A single molecule of water is the reason certain mutations are more responsive to a 'targeted' cancer therapy, according to molecular-scale simulations carried out at the A*STAR Bioinformatics Institute.
Targeted therapies that take down tumors by selectively interfering with the defective proteins that promote their survival and growth have revolutionized cancer therapy. For example, the drug afatanib is a targeted agent given to lung cancer patients with certain mutations in the gene encoding the epidermal growth factor receptor (EGFR). Recent clinical studies suggest that afatanib may confer survival benefit specifically in patients with the EGFR19del mutation rather than in patients with a different mutation, known as EGFRL858R, when compared with patients receiving only standard chemotherapy.
Daniel Shao-Weng Tan, a clinical oncologist from A*STAR's Genome Institute of Singapore and the National Cancer Center of Singapore, who participated in these trials in Singapore, was confounded by this disparity, so he consulted scientist Chandra Verma at the Bioinformatics Institute to rationalize their findings. Verma and Srinivasaraghavan Kannan, a specialist in molecular modeling at the Bioinformatics Institute, teamed up with Tan to identify differences in the structure and dynamic behavior of these two mutant proteins that might explain the distinct response profiles.
It turns out that the critical difference was a mere molecule of water. Afatanib binds to a pocket on EGFR that is missing five amino acids in the EGFR19del mutant. Molecular dynamics simulations carried out at the Bioinformatics Institute and the National Supercomputing Centre, Singapore, showed that this deletion creates a more physically constrained structure, which can snugly accommodate both afatanib and a single water molecule in a very stable arrangement. In contrast, the mutant EGFRL858R, also found to characterize lung cancer, contains just a single amino acid substitution, and is predicted to hold two water molecules in its pocket. However, these are in a weaker and relatively less stable arrangement, resulting in weakened interactions with afatanib that likely reduce the drug's affinity and hence effectiveness.
"Our hypothesis is based on understanding the physics of the system and could offer a compelling rationale for the clinical observations with afatanib," says Kannan. Intriguingly, he and Verma were also able to home in on other binding pocket mutations (single nucleotide polymorphisms, or 'SNPs') that occur alongside EGFR19del that might likewise influence the efficacy of afatanib.
The researchers are now examining whether these predictions hold in real-life experiments and see this as a promising general strategy for predicting patient response to targeted treatments. "We are trying to develop a robust pipeline to examine the structural effects of mutations on drug interactions," Kannan says. "This will be a very valuable approach to complement our engagement with the national precision medicine efforts."
More information: Srinivasaraghavan Kannan et al. Hydration effects on the efficacy of the Epidermal growth factor receptor kinase inhibitor afatinib, Scientific Reports (2017). DOI: 10.1038/s41598-017-01491-z