New glaucoma drug from a Duke University spinoff has an edge over other treatments
A 12-year-old Duke University spinoff founded by two Duke professors has received regulatory approval for its first drug: once-a-day eye drops for glaucoma patients.
Aerie Pharmaceuticals received approval for the medication, Rhopressa, from the Food and Drug Administration on Dec. 18. Rhopressa is designed for open angle glaucoma, the most common form of the degenerative eye disease, affecting about 3 million Americans. The eye drops, expected to be available to glaucoma patients by mid-2018, would be used in conjunction with another glaucoma medication.
The company called the FDA approval "a testament to years of successful research and development and the incredible talents of our dedicated employees."
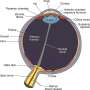
Glaucoma is a degenerative eye disorder without symptoms in the early stages, primarily affecting the elderly, with a higher incidence among African Americans. Glaucoma is caused by a buildup of fluid in the eye that results in increasing pressure on the optical nerve.
The progress of glaucoma can be slowed down by medication or surgery if detected early, but the condition can lead to vision loss if not caught in time.
Glaucoma is typically treated with eye drops that require up to four applications daily and have unpleasant side effects, such as redness, blurred vision, dry eyes and irritation. Rhopressa is used only once a day; its side effects may include pain, stinging and redness.
Aerie is developing a second product, Roclatan, which would provide all four of the known mechanisms for lowering interocular pressure in one medication. Roclatan would pair Rhopressa with another medication and would be marketed as a primary source of treatment for glaucoma, rather than an adjunct to another treatment. The company plans to apply for FDA approval for Roclatan next year.
Rhopressa is designed to permit the release of fluids by relaxing the main drainage chamber in the eye.
The company's next step is to persuade health insurance companies to pay for Rhopressa. Commercial insurers represent approximately half the drug's potential U.S. market. The other half of Rhopressa users would be covered under Medicare Part D. Aerie said it expects Medicare coverage for Rhopressa to begin in January 2019.
Aerie is still negotiating with health insurers and has not publicly announced a price for Rhopressa.
The company was co-founded in 2005 by the late David Epstein, who was a medical professor at Duke, and Eric Toone, the university's vice provost and director of its Innovation and Entrepreneurship Initiative. It is now based in Irvine, Calif., but still maintains a research and development operation in Durham.
Aerie does not disclose how many people it employs at local offices.
The company plans to hire 100 sales reps in the first quarter of 2018 to persuade doctors to prescribe the medication.
Aerie's website says Rhopressa and Roclatan represent a $5 billion market in the U.S., Europe and Japan, and the market is expected to exceed $8 billion by 2023.
©2018 The News & Observer (Raleigh, N.C.)
Distributed by Tribune Content Agency, LLC.