Defining the responsibility to recontact research participants with new genetic findings
The American Society of Human Genetics (ASHG), along with several co-signing organizations, issued a position statement today outlining whether, and to what extent, there is a responsibility to recontact genetic and genomic research participants when new findings emerge that suggest their genetic information should be interpreted differently, which would allow participants to benefit from current genomics advances. The statement was published in The American Journal of Human Genetics (AJHG).
As research progresses and new data emerge, scientific understanding of what it means to have a given genetic variant can evolve, the statement authors wrote. Such changes, if conveyed to research participants with that variant, can provide important information about their health and affect their medical care. ASHG and the science community deeply value the contributions of research participants, without whom many important advances would not have taken place, and share a desire for participants to learn about and benefit from the newest findings. At the same time, there are serious practical and logistical challenges to be considered in requiring researchers to monitor scientific literature for changes to clinical variant interpretations and to keep past and current participants apprised of such changes.
"While clinical recommendations on this topic have begun to emerge, there is a lack of guidance on the responsibility of researchers to inform participants of reinterpreted results," said Yvonne Bombard, Ph.D., former chair of the ASHG Social Issues Committee and co-lead author of the statement. "Because the research and clinical contexts have different goals, priorities, timelines, and restrictions, we need to consider them separately," she explained.
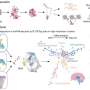
To meet this need, an ASHG-led workgroup including representatives from the National Society of Genetic Counselors, the Canadian College of Medical Genetics, and the Canadian Association of Genetic Counsellors assembled a position statement containing background information, factors to consider, and a set of 12 recommendations and flowchart for determining the extent of responsibility to recontact. The recommendations were endorsed or supported by the Genetic Alliance, the European Society of Human Genetics, the American Association of Anthropological Genetics, the Executive Committee of the American Association of Physical Anthropologists, the Human Genetics Society of Australasia, and all organizations in the workgroup.
The statement reports a variety of factors that would affect the strength of the responsibility to recontact, and concluded this responsibility is stronger when:
- The research is active, ongoing, has funding, and participant contact details are up-to-date
- The informed consent process set an expectation of potential contact or recontact
- There is high certainty about the new interpretation of the genetic variant
- The reinterpretation would be relevant to the condition being investigated
If the interpretation of a given variant is related to the condition under study or reasonably expected to affect participants' medical management, ASHG strongly recommends that researchers make reasonable attempts to recontact participants to offer updated results. If the reinterpretation is not expected to affect medical management, recontact is advised rather than strongly recommended.
Conversely, the statement recommends that there is no responsibility for researchers to hunt or scan the genomic literature for changes in variant interpretation, and that any responsibility to recontact should be limited to the duration of research funding. Additional recommendations address the practicalities of informed consent, involvement of institutional review boards, timeliness and protocol of recontact, and structuring of future research studies.
"Technological advances could help ease the practical challenges of recontacting participants, improving its feasibility for researchers," noted Howard Levy, MD, Ph.D., co-lead on the statement. Efforts to cross-reference and integrate research databases, as well as to create lay-friendly information and automated notifications of variant reinterpretation, could enable a more self-service model of educating research participants about continued research progress.
More information: Bombard Y et al. (4 April 2019). The responsibility to recontact research participants after reinterpretation of genetic and genomic research results. The American Journal of Human Genetics. 104: 578-595. DOI: 10.1016/j.ajhg.2019.02.025