Endotoxin shock protector
The bacterial toxin lipopolysaccharide (LPS) is one of the most potent virulence factors of Gram-negative bacteria that cause sepsis. Exposure to even tiny amounts can trigger a systemic—and potentially lethal—inflammatory response known as endotoxin shock.
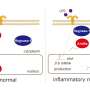
Proinflammatory SRTFs (stress-responsive transcription factors) and lipid-regulating SREBPs (sterol regulatory element binding proteins) drive nuclear signaling pathways that have been implicated in lethal endotoxin shock. SRTFs are ferried to the cell's nucleus by the adaptor protein importin alpha 5, whereas SREBPs are transported by importin beta 1.
Using a novel cell-penetrating peptide they designed and tested in mice that selectively suppresses nuclear transport through importin alpha 5, Jacek Hawiger, MD, Ph.D., Jozef Zienkiewicz, Ph.D., Yan Liu, MD, and colleagues showed that endotoxin shock is mediated by proinflammatory SRTFs rather than lipid-regulating SREBPs.
Their findings, reported last month in the journal ImmunoHorizons, highlight a new tool that could be applied to the mechanistic analysis of inflammation due to other microbial as well as allergic, autoimmune, metabolic or physical insults.
More information: Yan Liu et al. Protection from Endotoxin Shock by Selective Targeting of Proinflammatory Signaling to the Nucleus Mediated by Importin Alpha 5, ImmunoHorizons (2019). DOI: 10.4049/immunohorizons.1900064