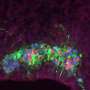
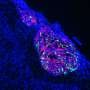
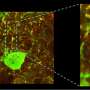
Like a molecular knob: How a gene controls the electrical activity of the brain
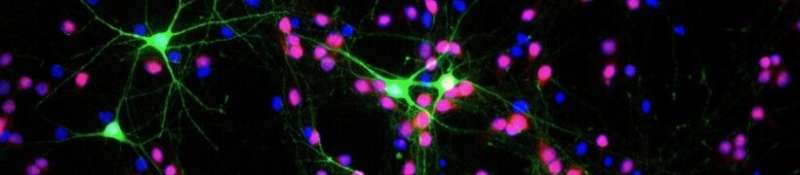
It works like a very fine "molecular knob" able to modulate the electrical activity of the neurons of our cerebral cortex, crucial to the functioning of our brain. Its name is Foxg1, it is a gene, and its unprecedented role is the subject of the discovery just published in the journal Cerebral Cortex. Foxg1 was already known for being a "master gene" able to coordinate the action of hundreds of other genes necessary for the development of our anterior central nervous system. As this new study reports, the "excitability" of neurons, namely their ability to respond to stimuli, communicating among each other and carrying out all their tasks, also depends on this gene.
To discover this, the researchers developed and studied animal and cellular models in which Foxg1 has an artificially altered activity: A lack of activity, as it happens in patients affected by a rare variant of Rett Syndrome, which leads to clinical manifestations of the autistic realm; or an excessive action, as in a specific variant of the West Syndrome, with neurological symptoms such as serious epilepsy and severe cognitive impairment. As deduced by the scientists in the research, the flaw in the "knob" lies in altered electrical activity in the brain with important consequences for the entire system, similar to what happens in the two syndromes mentioned.
Shedding light on this mechanism, say the researchers, allows an understanding of how much more deeply the functioning of our central nervous system is in sickness and in health, a fundamental step to assess possible future therapeutic interventions for these pathologies. What has just been published is the latest in a series of three studies on the Foxg1 gene, recently published by the researchers of SISSA, in Cerebral Cortex. It is the result of a project begun more than five years ago, which saw the team of Professor Antonello Mallamaci of SISSA on the front line with researchers of the University of Trento and the Neuroscience Institute of Pisa, with the support of the Telethon Foundation, of the Fondation Jerome Lejeune and of the FOXG1 Research Foundation.
The many abilities of the "master gene"
"We know that this gene is important for the development of the anterior central nervous system," explains the Professor Antonello Mallamaci of SISSA, who has coordinated the research. "In previous studies we had already highlighted how it was involved in the development of particular brain cells, the astrocytes, as well as the neuronal dendrites, which are part of the nerve cells that transport the incoming electrical signal to the cell. The fact that it had mutated in patients affected by specific variants of Rett and West Syndromes in which we see, respectively, an insufficient and excessive activity of this gene, made us explore the possibility that there was also another role. And, from what has emerged, it would appear that way."
The research findings
According to the study, the activation of the electrical activity of Foxg1 follows a positive circuit. Professor Mallamaci explains: "If the gene is very active there is increased electrical activity in the cerebral cortex. In addition, the neurons, when active, tend to make it work even harder. One process, in short, feeds the other. Obviously, in normal conditions, the system is slowed down at a certain point. "If, however, the gene functions abnormally, or it is found in a number of copies other than two, as happens in the two syndromes above, the point of balance changes and the electrical activity is altered. All this, in addition to making us understand the mechanisms of the pathology, tells us that Foxg1 functions precisely as a key regulator of the electrical activity in the cerebral cortex."
The next step, explains the professor, will be to understand the role of the mediating genes, namely of some of the many genes whose action is regulated by the master gene Foxg1. This analysis is important to understand in more detail how this gene works under normal and pathological conditions.
How the master gene produces the pathological effects, when and how to intervene
Understanding the molecular mechanisms that Foxg1 controls is also important to study what could be the targets on which to intervene for possible therapeutic approaches. "Given that finding a therapy for these illnesses is very difficult, working so in depth you might find, for example, that most problems are caused precisely by some of the "operators" that Foxg1 regulates. And that we should therefore focus our attention on these goals, rather than on the master gene, maybe using drugs that already exist and have been seen to be useful in remedying those specific flaws."
In the case of a future approach that would instead correct the anomalies of the FOXG1 gene with the gene therapy, explains Professor Mallamaci, "it is necessary to understand when to intervene, namely from what moment on the pathological effects due to the mutation of this gene become irreversible. To replace the flawed copy with the correct one, it is necessary to intervene before that moment, which might suppose you would have to make a prenatal gene diagnosis and treatment. The next steps we will take," concludes Professor Mallamaci, "will be directed precisely in the direction of a deeper understanding of all these aspects."
More information: Wendalina Tigani et al, Foxg1 Upregulation Enhances Neocortical Activity, Cerebral Cortex (2020). DOI: 10.1093/cercor/bhaa107