Biologists discover super-enhancers that switch on breast cancer genes
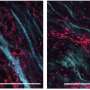
Triple-negative breast cancer (TNBC) is an aggressive type of breast cancer with a high fatality rate. Currently, chemotherapy is the major treatment option, but the clinical result is unsatisfactory. A research team led by biologists at City University of Hong Kong (CityU) has identified and characterized a set of specific super-enhancers that stimulate the activity of the related critical cancer genes. The research has also discovered that the deletion of certain specific super-enhancers can reduce tumor cell growth. The latest findings may help discover new effective drug targets for TNBC patients to improve their survival chance.
Traditionally, cancer research has been focused on identifying gene mutations in different types of breast cancer. In contrast, how the epigenetic circuit affects cancers remains poorly characterized.
Epigenetic change: another way to induce cancers
While genetic mutation is a change in one or more parts of the DNA sequence, an epigenetic change also changes a gene's DNA, but not at the sequence level. Instead, special marks, called epigenetic marks, are added to or removed from the DNA sequence to change how a protein works in the body. And specific epigenetic marks are contained in super-enhancers. Deregulation, meaning abnormal regulation, of super-enhancers can therefore induce high production of cancer driver proteins and promote cancer formation.
To find out how these super-enhancers can affect TNBC cells, Dr. Rebecca Chin Yuet-ming, a cancer biologist, and Dr. Wang Xin, a computational biologist, both from the Department of Biomedical Sciences at CityU, joined hands together to lead the study. Their findings were published in the scientific journal Nature Communications, titled "Defining super-enhancer landscape in triple-negative breast cancer by multiomic profiling."
Integrating multi-level epigenomic sequencing data for 21 cell lines with gene expression data and clinical information for over 4,000 patient samples, Dr. Wang's team used the method of multiomic profiling to perform in-depth data mining and built a specific super-enhancer-target regulatory network for all types of breast cancer.
A key regulator of cancer growth
"Our integrated analyses reveal that the clustering of super-enhancers is sufficient to characterize different subtypes of breast cancer," said Dr. Wang. "Importantly, based on the regulatory network, we identify the gene FOXC1 as a key regulator of cancer growth and metastasis which is driven by a TNBC-specific super-enhancer. The FOXC1 is predictive of patients' survival and help develop therapeutic strategies targeting epigenetic circuits."
A number of key cancer driver genes (oncogenes), including FOXC1 and MET, are known to promote cancer growth and are associated with worse survival in TNBC patients. However, very little is known about how these genes being specifically highly expressed in TNBC. "Our network biology analysis uncovers FOXC1 as the master regulator of a large set of genes in metastasis. Using CRISPR/Cas9 technology, we further directly demonstrate that super-enhancer drives FOXC1 expression, and importantly, enhances cancer growth in mouse models," said Dr. Chin.
By performing analysis on in-house clinical samples, the teams also learnt that FOXC1 upregulation is associated with higher tumor grade, cell division rate, and tumor-infiltrating immune cells.
Another TNBC-specific gene
The researchers went a step further and applied the integrated method to discover another new TNBC-specific gene ANLN. ANLN has been shown to be correlated with TNBC recurrence and poor survival rate in previous studies. In this study, the team found that the deletion of super-enhancer of ANLN could reduce the protein expression and tumor cell growth. "These findings demonstrate the power of leveraging epigenetic landscape to identify novel players in TNBC, paving the way to discover more effective therapeutic targets for this aggressive form of breast cancer," Dr. Chin said.
Breast cancer is the most common type of cancer in women, with about 4,600 new cases of breast cancer being diagnosed in Hong Kong each year. Among all breast cancers, TNBC accounts for about 10-15%. Different from other types of breast cancer, TNBC does not express hormone receptors (namely estrogen receptors and progesterone receptors) and a protein called Her2, and that's where its name "triple-negative breast cancer" comes from. So TNBC is quite "invisible," and it is also difficult to heal as the scientists have not found an effective "target" on the TNBC cancer cell for the drugs to attack.
TNBC usually develops between the ages of 40-50, younger than the average breast cancer patients. Also, the time for TNBC metastasis is shorter. Patients typically relapse within 5 years of treatment, and is considered to have a poorer prognosis than other breast cancer forms.
"We hope our findings can contribute to the development of effective drugs for TNBC patients to improve their chance of survival," said Dr. Chin.
More information: Hao Huang et al, Defining super-enhancer landscape in triple-negative breast cancer by multiomic profiling, Nature Communications (2021). DOI: 10.1038/s41467-021-22445-0