A rapid method to quantify SARS-CoV-2 antibodies
Scientists have developed a rapid, highly-accurate test to detect antibodies against the spike protein of SARS-CoV-2 in human serum, opening a new avenue for understanding the full extent of the pandemic and evaluating the effectiveness of vaccines.
In the 18 months since the emergence of COVID-19 pandemic, great strides have been made in discovering and inventing various approaches to track and control the spread of the SARS-CoV-2 virus. Rapid and accurate diagnosis has always been vital in this regard. The gold standard since the beginning of the pandemic has been the RT-PCR method; however, it is time-consuming, labor-intensive, and requires sophisticated equipment, and can only detect the presence of viral RNA in the samples.
A team of scientists from Japan, including Professor Manabu Tokeshi of Hokkaido University's Faculty of Engineering, have developed a 20-minute test to detect and quantify antibodies against SARS-CoV-2 in human serum. Their findings were published in the journal Biosensors and Bioelectronics.
It is estimated that between 40% to 45% of individuals infected with SARS-CoV-2 are asymptomatic—despite being infected, they do not develop any symptoms of the disease. Identifying individuals who may have had asymptomatic COVID-19 is important to understanding the full extent of the pandemic. RT-PCR can only detect the presence of the viral RNA in samples; individuals who have recovered from the pandemic will only have antibodies to the virus, which RT-PCR cannot detect.
There are commercially available tests to detect the presence of antibodies against SARS-CoV-2; however, the majority of these tests are inaccurate. Lateral flow immunoassay is one of the most common tests for diagnosis of COVID-19 based on antibody detection. While rapid, it is a qualitative method, and cannot be used for the quantification of antibodies. Further, the method does not scale well, resulting in a low cost efficiency.
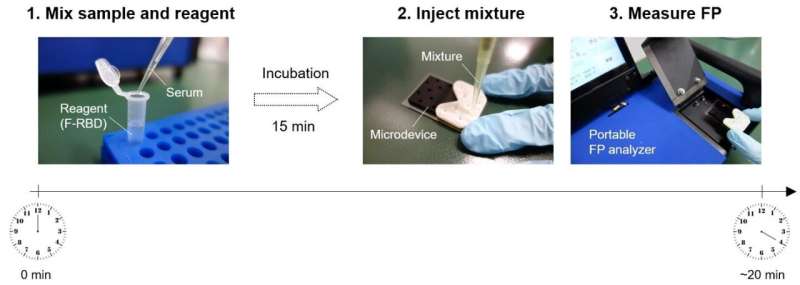
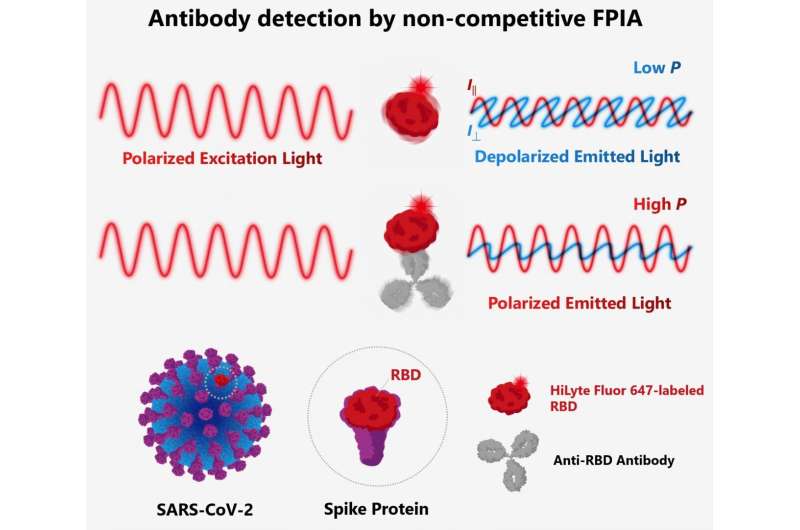
The scientists developed a method of detecting antibodies against SARS-CoV-2 in human serum using a method called non-competitive fluorescence polarization immunoassay (FPIA). This method is not just rapid, but can also be used to quantify the antibodies. FPIA has been used in the food and medical industry; the team has previously made many innovations to the method and associated equipment, including the development of a portable fluorescence polarization analyzer.
FPIA requires fluorescently-labeled recombinant SARS-CoV-2 spike proteins (F-RBD) and human serum to be mixed together for the test. Individuals who have been infected with or vaccinated against SARS-CoV-2 will have anti-spike protein antibodies in their serum. When these antibodies bind to F-RBD, polarized light is emitted, while F-RBD alone emits depolarized light. By measuring the degree of polarization using a fluorescence polarization analyser, the concentration of antibodies can be determined. The scientists optimized the test and evaluated it using samples of human serum from individuals diagnosed with COVID-19 and from those who had not been infected by SARS-CoV-2.
The test was demonstrated to be highly accurate, quick and easy to perform, with high throughput. This test requires about 20 minutes to complete, compared to about 2 hours for other tests; furthermore, the equipment required for the test is highly portable, weighing only 4.3 kg. Taken all together, these features make the test an excellent option for detecting and quantifying antibodies against SARS-CoV-2. The test can now be used for two purposes: screening large populations to determine the exact extent of the pandemic, and from evaluating the effectiveness of SARS-CoV-2 vaccines based on the antibody response.
More information: Keine Nishiyama et al, Facile and rapid detection of SARS-CoV-2 antibody based on a noncompetitive fluorescence polarization immunoassay in human serum samples, Biosensors and Bioelectronics (2021). DOI: 10.1016/j.bios.2021.113414