Blueprint for regulating lab-developed diagnostic tests
How should diagnostic tests developed in laboratories in hospitals and other health care settings be regulated—if at all? That's a question that has stirred lively debate within the U.S. health care system for years, but certain temporary deviations in Food and Drug Administration (FDA) policy in response to the COVID-19 pandemic may offer a blueprint for regulatory oversight of laboratory-developed tests (LDTs), according to a new study by researchers at Massachusetts General Hospital (MGH) published in the Journal of Molecular Diagnostics. This research provides concrete data that suggests what FDA regulation of LDTs might look like. This data could help inform pending legislation aiming to change the regulatory oversight of certain "high risk" LDTs.
Diagnosing many human diseases relies on in vitro diagnostic (IVD) tests, which include tests performed in a test tube, culture dish, or elsewhere outside a living organism. (IVDs are also sometimes called in vitro clinical tests, or IVCTs.) The FDA already closely regulates commercially available IVDs, requiring manufacturers to submit data for premarket approval before they can be sold. However, clinical laboratories located in hospitals and other health care settings can create their own IVDs for in-house use, which are known as LDTs. Historically, the FDA has exercised little oversight of LDTs (so-called enforcement discretion).
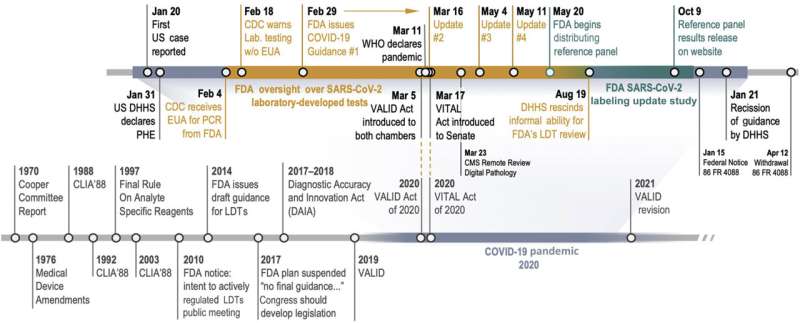
However, that policy changed with the onset of the COVID-19 pandemic, notes Jochen Lennerz, MD, Ph.D., medical director of the MGH Center for Integrated Diagnostics (CID) and the study's senior author. In March 2020, the FDA asserted authority over LDTs, requiring labs that produced them for detecting COVID-19 to undergo emergency use authorization (EUA). Requiring makers of LDTs to submit validation data to enable assessment of performance and accuracy of their assays was the first time the FDA took concrete steps to regulate in-house diagnostic tests, a significant deviation from policy.
That requirement was later rescinded and labs were once again allowed to administer LDTs without FDA authorization. However, the FDA required the makers of LDTs that had received EUAs and makers of manufactured tests to validate their products' accuracy by running them on a reference panel that served as a gold standard for detecting COVID-19. This so-called labeling update study was a second deviation from FDA policy regarding regulation of LDTs.
Using data from their own lab, as well as from facilities around the nation, Lennerz and his colleagues analyzed the impact of these two deviations and found several key takeaways:
- Despite concerns that the FDA wasn't responding fast enough, a timeline of the initial 14 tests to receive EUAs showed that the process took 17 days, on average, from submission to authorization.
- Validating an IVD's accuracy with the FDA's reference panel carries costs. A typical lab required about 14 hours of technician time to complete the process. Add materials and other expenses to labor costs and each lab spent between $1,800 and $7,800 in the labeling update study.
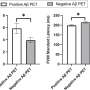
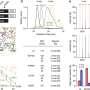
- IVDs for COVID-19 weren't as sensitive as their makers initially reported. The labeling update study—which used a common reference standard known as limit of detection (LOD)—found the true LODs to be significantly higher for most of the tests, from both labs and manufacturers.
Yet there's critical data that the labeling update study did not measure, notes Lennerz. Manufacturers submitted data about the performance of their IVDs based on their own in-house testing. But how well do manufacturers' IVDs perform in the real world, when they're used in hospital pathology labs? "The quality of a diagnostic test relies heavily on the competence of people," says Lennerz. "We all make errors from time to time. What the performance of manufacturers' tests looks like routinely and across clinical laboratories remains a question mark. And we currently do not have the tools to assess this systematically."
However, one of two bills that Congress is considering, the Verifying Accurate Leading-edge IVCT Development (VALID) Act, proposes mechanisms to expand regulatory oversight by the FDA. In one of these proposed mechanisms, known as technology certification, a laboratory would submit data about the design and performance of the highest-complexity test that it performs to the FDA, which would conduct a detailed review. This test would serve as a representative for the lab's entire design-validation process. In contrast, the competing bill (known as the VITAL Act) does not provide such mechanisms or details. VALID proposes to place responsibility for oversight of high-risk LDTs in the hands of the FDA, which Lennerz feels is the right choice. "I think the FDA has the appropriate standing to obtain comprehensive comparison data and help establish tools to regulate complex diagnostic tests," he says.
More information: Hetal D. Marble et al, Temporary Regulatory Deviations and the Coronavirus Disease 2019 (COVID-19) PCR Labeling Update Study Indicate What Laboratory-Developed Test Regulation by the US Food and Drug Administration (FDA) Could Look Like, The Journal of Molecular Diagnostics (2021). DOI: 10.1016/j.jmoldx.2021.07.011