New evidence identified on safety of IVF embryo screening method
Researchers at the University of Kent have identified crucial new evidence on the safety and efficacy of a controversial area of IVF treatment—preimplantation genetic testing for aneuploidy (PGT-A).
PGT-A is the screening for gross genetic (chromosomal) abnormalities in human IVF embryos with a view to improving IVF success rates and guarding against miscarriage. The UK Human Fertilisation and Embryology Authority's (HFEA) however recently assigned it a "red light" in terms of its safety and efficacy. The current study provides strong evidence for the benefits of PGT-A.
The, researchers used the HFEA's own 2016-2018 data to examine live birth and other outcomes reported, with and without PGT-A.
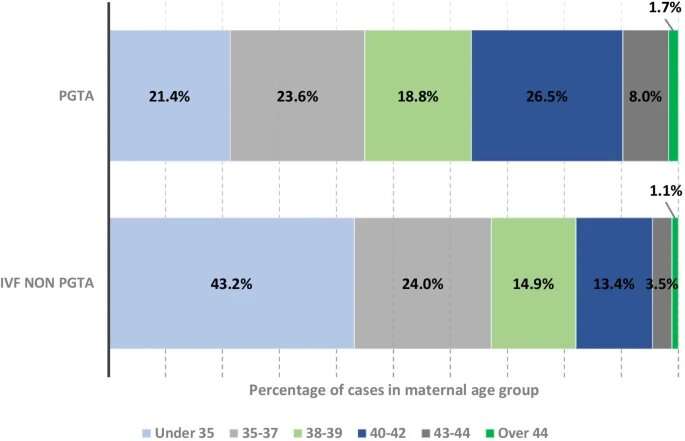
Statistical analysis of differences between PGT-A and "regular" IVF cycles was performed, grouping by maternal age, the leading known risk factor for chromosome abnormalities.
Data was gathered for nearly 2,500 PGT-A, out of a total of over 190,000 cycles in total. Live Birth Rate per embryo transferred and per treatment cycle was significantly higher for all PGT-A vs regular IVF age groups. In patients aged over 40, the reported live birth rates were 3-11 times greater when using PGT-A compared to regular IVF alone.
There was also a reduced number of transfers per live birth, particularly for women aged over 40, implying patients became pregnant faster if PGT-A was used.
Though the study identified strong evidence-based benefits of PGT-A, it is limited in matching with complete clinical indication information, PGT-A and non PGT-A cohorts
PGT-A is a controversial process in IVF. Proponents argue that evidence suggests this treatment is effective and safe. Opponents argue that, until randomized clinical trials establish this unequivocally, patients should not be subjected to it; especially as this is a paid-for treatment.
On the basis of a traffic light system designed to assess the suitability of adjunct treatments for IVF, the HFEA previously assigned the process two Red Lights as a treatment whose efficacy and safety had not been established. This was recently changed to one red light.
Darren Griffin, Professor of Genetics at Kent and Lead Author on the paper said: 'This data will hopefully aid the HFEA in their continual surveillance of the "Red traffic light" guidance that currently states there is no evidence that PGT-A is effective or safe. The guidance could be revised in the light of this new data for patient benefit. I appreciate the collegial way in which the HFEA have assisted in providing this data and their open-mindedness to the prospect of revisiting their guidance and traffic light system.'
The paper is published in the Journal of Assisted Reproduction and Genetics.
More information: Kathryn D. Sanders et al, Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK Human Fertilisation and Embryology Authority data collection 2016–2018, Journal of Assisted Reproduction and Genetics (2021). DOI: 10.1007/s10815-021-02349-0