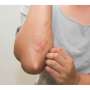
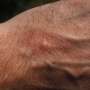
FDA approves Rinvoq for treatment of atopic dermatitis
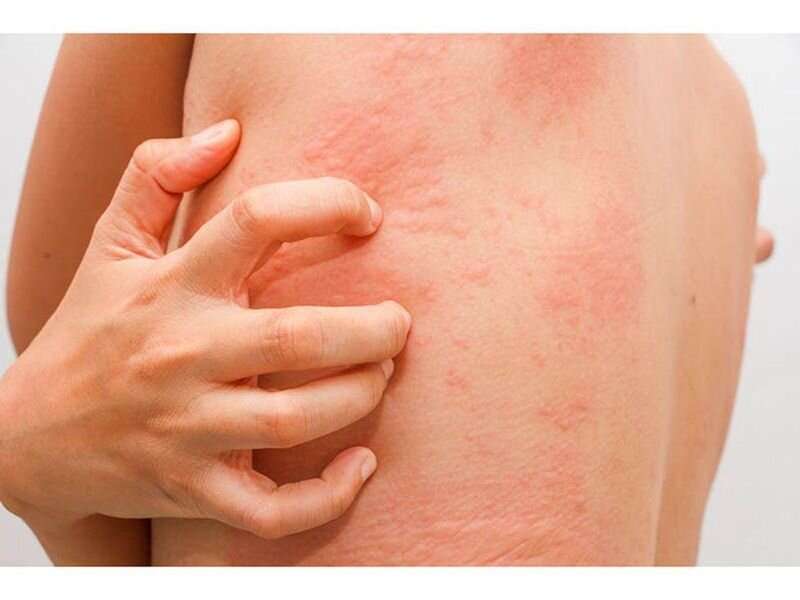
The U.S. Food and Drug Administration approved Rinvoq (upadacitinib) for the treatment of moderate-to-severe atopic dermatitis in patients aged 12 years and older, the manufacturer announced Friday.
The approval is indicated for patients with atopic dermatitis that did not respond to previous treatment and is not well controlled with other medications or when the use of other medications is not recommended. A dose of 15 mg Rinvoq can be initiated in patients 12 years and older who weigh at least 40 kg (88 lb); in those younger than 65 years of age who do not achieve an adequate response at this dose, the dose can be increased to 30 mg once daily.
Approval was based on efficacy and safety data from more than 2,500 patients in three studies. Approximately 52 percent of these patients had previous exposure to systemic atopic dermatitis treatment. Across the three studies at 16 weeks, patients receiving once-daily Rinvoq (15 mg and 30 mg) monotherapy and combined with topical corticosteroids met the primary end points of at least a 75 percent reduction in the Eczema Area and Severity Index and a validated Investigator Global Assessment for Atopic Dermatitis of clear or almost clear (0/1) with at least two grades of reduction from baseline. A significant improvement in Worst Pruritis Numerical Rating Scale ≥4 was seen as early as the first week of treatment with Rinvoq compared with placebo.
The safety profile of Rinvoq in patients with atopic dermatitis was similar to that seen in patients with rheumatoid arthritis. Other adverse reactions specific to patients with atopic dermatitis included eczema herpeticum/Kaposi's varicelliform eruption. Serious side effects of Rinvoq include infections such as tuberculosis, a greater risk for death in people ages 50 years and older who have at least one cardiovascular risk factor, an increased risk for certain cancers, and blood clots.
Approval was granted to AbbVie.
More information: More information is available here.
2022 HealthDay. All rights reserved.