New progesterone-based formulations show promise for the treatment of retinitis pigmentosa

Retinitis pigmentosa is a degenerative disease affecting the photoreceptor cells in the retina, known as cones and rods. Of genetic origin, this disease first affects vision in low light conditions, and progressively peripheral vision and the central field of vision until total sight loss occurs, as the photoreceptor cells gradually die. Noting recent research into the role of hormones, particularly progesterone, in preventing cell death due to oxidative stress, the CEU UCH Drug Delivery Systems (DDS) research group has successfully developed and tested a range of methods of delivery of this hormone into the eye to slow the degenerative process characteristic of retinitis pigmentosa.
The research undertaken by the team is detailed in Dr. Adrián Alambiaga's doctoral thesis, supervised by Professor Alicia López Castellano and Dr. Aracely Calatayud, and which he successfully defended at CEU in April. His findings, already published in several journals, with the latest being Pharmaceuticals, show that progesterone can be delivered in various forms, such as eyedrops, micelles and inserts, in sufficient quantity to penetrate the surface of the eye and reach the neuroretina without causing significant toxicity or irritation.
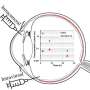
Dr. Adrián Alambiaga said, "The use of ocular inserts enables progesterone to be delivered in greater quantities than when aqueous solutions are used. Although we saw that aqueous solutions can also permeate the sclera and the cornea, inserts performed best in our tests, as they liberate progesterone for absorption by the neuroretina over a sustained period and in greater quantity."
Progesterone is virtually insoluble in water and it only dissolves in gastrointestinal fluids slowly and incompletely. This means that when it is administered orally, it quickly becomes ineffective. In addition, high levels of progesterone need to be administered orally so that just a small amount can reach the eye. "That's why it was important to study the different ways of administering progesterone topically or locally in the eye, thereby limiting the amount that has to be administered."
Over the course of his doctoral research, Dr. Alambiaga, under the supervision of Prof López Castellano and Dr. Calatayud, developed a range of pharmaceutical formulations of progesterone for topical delivery to the eye. These included aqueous solutions, which increase the durability and diffusion of the molecules on the ocular surface, and ocular inserts, which increase the contact time of the drug on the ocular surface, increase drug availability to the body, and enable a controlled release, more precise dosages and less frequent administration.
For the group's lead researcher, Professor Alicia López Castellano, who specializes in pharmaceutical technology at CEU UCH, Dr. Alambiaga's thesis shows that "we have demonstrated for the first time that topical administration of progesterone in the eye is viable. This opens up possible new therapeutic strategies for retinitis pigmentosa patients, and by extension for patients with other eye conditions in which oxidative stress is a risk factor, such as glaucoma, age-related macular degeneration, macular oedema due to retinal vein occlusion, cytomegalovirus retinitis, posterior uveitis and diabetic retinopathy."
More information: Adrián M. Alambiaga-Caravaca et al, Micelles of Progesterone for Topical Eye Administration: Interspecies and Intertissues Differences in Ex Vivo Ocular Permeability, Pharmaceutics (2020). DOI: 10.3390/pharmaceutics12080702
Adrián M. Alambiaga-Caravaca et al, HPLC-UV analytical validation of a method for quantification of progesterone in ex vivo trans-corneal and trans-scleral diffusion studies, Journal of Pharmaceutical and Biomedical Analysis (2020). DOI: 10.1016/j.jpba.2020.113749
Adrián M. Alambiaga-Caravaca et al, Development, characterization, and ex vivo evaluation of an insert for the ocular administration of progesterone, International Journal of Pharmaceutics (2021). DOI: 10.1016/j.ijpharm.2021.120921
Adrián M. Alambiaga-Caravaca et al, Topical Ocular Administration of Progesterone Decreases Photoreceptor Cell Death in Retinal Degeneration Slow (rds) Mice, Pharmaceuticals (2022). DOI: 10.3390/ph15030328