Researchers identify cells that cause neuronal death in a mitochondrial disease animal model
Microglia, a type of central nervous system cell, is primarily responsible for neuronal death in Leigh Syndrome and the neurological symptoms related to this mitochondrial disease. This is the conclusion of a research group from the Institut de Neurociències of the Universitat Autònoma de Barcelona (INc-UAB) in a new study carried out on a mouse model of the disease. The study was coordinated by Juan Hidalgo, researcher at the INc and the Department of Cell Biology, Physiology and Immunology of the UAB, and published in the journal Glia.
Leigh Syndrome is the most common form of mitochondrial disease. The cause of this group of diseases is a malfunction of the mitochondria—the organelles responsible for generating energy for the cell to function properly—caused by mutations in mitochondrial RNA or cellular DNA. In Leigh Syndrome, organs and tissues that need more energy, such as muscles or the brain, cannot function normally, causing very serious motor and respiratory problems in people who suffer from it.
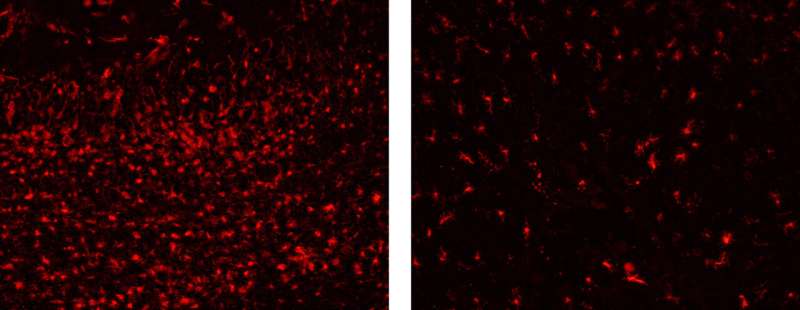
Previous studies had described high neuroinflammation in the brains of Leigh Syndrome mouse models, but its effect on the development of the disease was unknown: protective, harmless, or harmful. In the present study, researchers found that inflammation causes neuronal death and that the main element responsible for this process is microglia cells. These cells, which under normal conditions defend the nervous system from external or internal aggression, attack neurons with mitochondrial dysfunction, causing their death.
In the study, researchers analyzed the effect of suppressing microglial cells using a drug, Pexidartinib (PLX-3397). "With the removal of microglia, it took longer for motor problems to appear, and life expectancy was higher. In addition, when studying their brains, we found that there was much less neuronal loss," says Kevin Aguilar, first author of the article. "This drug, although it is not a good candidate for treating the disease, has been a key tool in identifying the effect of the neuroinflammation process and understanding how neuronal loss occurs," he adds.
The study also looked at the role of IL-6, a protein that modulates inflammatory activity and guides the activity of microglia. "We suspected that this protein would also play a key role in the symptoms of the syndrome. That's why we wanted to analyze what happened when there was a deficiency. However, contrary to what we expected, although respiratory problems were reduced, the effects were very moderate. This makes us think that there are other proteins involved," explains Aguilar. "Our next goal will be to describe the specific process by which microglia cells attack neurons and thus be able to develop more specific and selective treatments in the future," he concludes.
The study, which is a collaboration between the Neuroinflammation and Oxidative Stress research group and the Mitochondrial Neuropathology laboratory, both at the INC-UAB, is fundamental to understanding Leigh Syndrome and finding a therapy for mitochondrial diseases, which affect 1 in every 5,000 newborns.
More information: Kevin Aguilar et al, Microglial response promotes neurodegeneration in the Ndufs4 KO mouse model of Leigh syndrome, Glia (2022). DOI: 10.1002/glia.24234