3D organoid models identify potential treatment targets for a devastating pediatric kidney disease
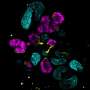
![CDH1+ distal nephron dilates in PKHD1−/− kidney organoids cultured under flow.(A) Schematic of kidney organoid-on-a-chip (flow) culture and static culture. (B) Diagram of differentiation days and culture conditions for flow and static culture. (C) Representative phase-contrast images of PKHD1−/− and PKHD1+/− organoids cultured under flow on days 16 to 18, days 21 to 25, and day 32 of differentiation. Red arrows highlight dilated tubules. Scale bars, 100 μm (top). Representative phase-contrast images of PKHD1−/− and PKHD1+/− organoids cultured under flow on day 35 of differentiation. White arrows highlight dilated tubules. Dashed white lines highlight normal tubules. Scale bar, 50 μm (bottom). (D) Immunocytochemistry with sample clearing for proximal tubule (LTL and CFTR) and distal tubule (CDH1) in PKHD1−/− organoids with and without flow on day 35. Scale bar, 100 μm. (E) Bright-field images of patient-derived ARPKD organoids with and without forskolin treatment in 3D culture on day 66. Scale bar, 250 μm (top). Immunocytochemistry for proximal tubule (LTL), distal tubule (CDH1), and podocyte (PODXL) in PKHD1−/− organoids with and without forskolin treatment on day 35. Scale bar, 100 μm (bottom). (F) Tubular diameters in nephron segments [proximal tubule (LTL) and distal tubule (CDH1)] in PKHD1−/− organoids with and without flow (left) and with and without forskolin treatment (right). Values represent means ± SD. **P < 0.01, ****P < 0.0001. Credit: Science Advances (2022). DOI: 10.1126/sciadv.abq0866 3D organoid models identify potential treatment targets for a devastating pediatric kidney disease](https://scx1.b-cdn.net/csz/news/800a/2022/3d-organoid-models-ide.jpg)
Organoids—lab grown cells or tissues that resemble organs—serve as a new tool for disease modeling, but researchers often have difficulty replicating the biophysical conditions in which the organs operate within the body.
This is especially true for modeling human diseases that require stimuli from cell microenvironments.
A research team from Massachusetts General Hospital, Brigham and Women's Hospital, the Wyss Institute and colleagues recently united organoids with organ-on-a-chip technology to replicate the unique disease process underlying autosomal recessive polycystic kidney disease (APRKD).
In a recent study in Science Advances, the team, which was led by Ken Hiratsuka, MD, Ph.D., and Ryuji Morizane, MD, Ph.D., reports using the new modeling system to identify two potential therapeutics for APRKD, which currently has no FDA-approved treatment.
APRKD is a disorder characterized by the formation of cysts in the kidneys, which enlarges the organs and causes the progressive loss of renal function. It has reported mortality rate as high as 30% in infancy. For those patients who survive, 41% will require a kidney transplant by the age of 11.
The causative gene for the disease is PKHD1, but previous attempts to model the disease in genetically modified mouse models have been unsuccessful. Researchers have been able to cultivate mutated PKHD1 cells in the lab. However, modeling the disease in a static 3D organoid does not work because it is caused by a mutation on the surface of the cells that is stimulated by urinary flow.
To overcome this challenge, the team used a 3D printer to create a perfusion chip that models the microenvironment of the cells within the kidney and allows liquid to flow through the organoids. By doing so the team identified two mechanosensing molecules (FOS and RAC1) that are potential therapeutic targets for the disease.
They also shed light on two important questions regarding the disease mechanisms of ARPKD:
- That the molecule FOS may be a crucial determent of species-specific cyst formation, which explains why mouse models were unable to effectively replicate the disease
- Why mutations in the PKHD1 gene lead to cyst formation
The team also tested two FDA-approved drugs (R-Naproxen and R-Ketorolact) that inhibit RAC-1 and one investigational new drug that inhibits FOS (T-5224), which were all shown to have therapeutic effects in these models.
Clinical trials will now be needed to investigate these therapeutics in patients with ARPKD. The success of the organoid modeling system in replicating the disease could also help researchers identify more potential treatment targets.
"In this study, we showed that our kidney organoids on a chip platform provides a physiologically relevant model for ARPKD, allowing the identification of mechanosensing signals as key drivers of cystogenesis," says Morizane, an investigator in the Division of Neurology at Mass General and Assistant Professor of Medicine at Harvard Medical School.
"In validation of our findings, FDA-approved NSAIDS that inhibit RAC1 as well as a clinically tested inhibitor of FOS are shown to have therapeutic effects in our model. Our observations highlight the vast potential of organoid-on-a-chip models to elucidate complex disease mechanisms for therapeutic testing and discovery."
More information: Ken Hiratsuka et al, Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery, Science Advances (2022). DOI: 10.1126/sciadv.abq0866