New test can ID patients at risk of severe COVID-19, study finds
A genomic test being developed by a Charlottesville, Va., company can predict a patient's risk of developing severe COVID-19, new research from UVA Health suggests. That information could help doctors identify patients at high risk for poor outcomes and quickly begin tailored treatment.
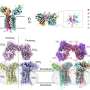
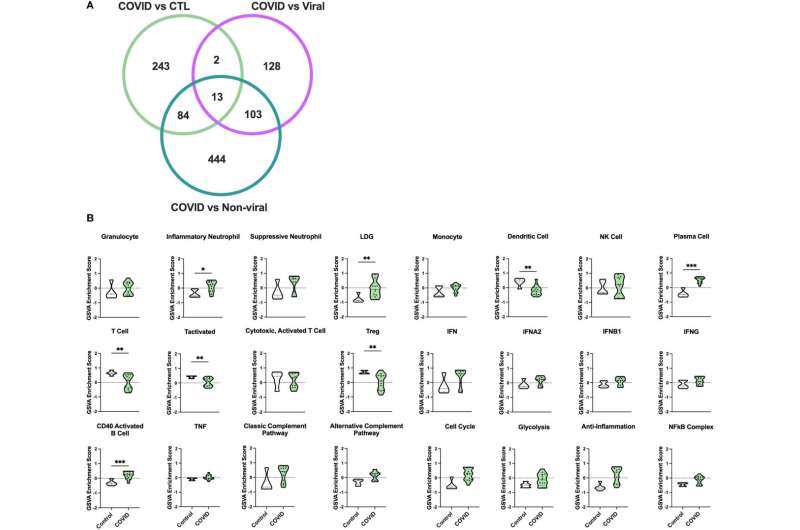
The approach proved more than 90% accurate at predicting outcomes among more than two dozen patients in the intensive care unit at UVA and 100 patients from publicly available data generated at Duke and Harvard. The test, called CovGENE, analyzes genes expressed in a person's blood to determine whether they may experience a severe disease course with increased risk of death.
"We have come far in the prevention and treatment of COVID-19 in the past two years. Regardless, we still struggle to identify patients at highest risk for severe disease. Our study uses a gene-analysis approach to identify an immune cell signature, distinct from other respiratory illnesses, that correlates with worse outcomes," said researcher Alexandra Kadl, MD, of UVA Health's Division of Pulmonary and Critical Care Medicine.
"This knowledge has the potential to help evaluate patients' immune profile with commonly, readily available assays to identify patients at risk for bad outcomes who would benefit from closer monitoring and advanced therapies to aid their recovery."
Predicting COVID-19 Severity
Based on the promising results of the UVA research, CovGENE's developer, AMPEL Biosolutions, is seeking to partner with a diagnostic testing company or pharmaceutical company to bring the approach to market as a simple PCR-based blood test.
"This unique collaboration with our colleagues from the University of Virginia has provided an easy and novel means to assess an individual patient's response to the SARS-CoV-2 virus and predict the clinical outcome," said Peter Lipsky, MD, AMPEL's CEO, chief medical officer and co-founder.
"Now that this unique approach has been validated, we look forward to its rapid development as a precision-medicine tool that can improve the outcome of patients with COVID-19 and reduce the number of hospitalizations, especially the most vulnerable."
The researchers have published their findings in the journal Frontiers in Immunology.
More information: Andrea R. Daamen et al, COVID-19 patients exhibit unique transcriptional signatures indicative of disease severity, Frontiers in Immunology (2022). DOI: 10.3389/fimmu.2022.989556