Influencing factors in SARS-CoV-2 nucleic acid detection
The COVID-19 epidemic continues to ravage the world. Various influencing factors in SARS-CoV-2 nucleic acid detection (NAT) and the significance of quantitative and qualitative detection capabilities deserve to be discussed, and several factors for improving testing capacity are proposed in a new article in Biosafety and Health to provide guidance for containing the spread of the outbreak.
Some institutions have carried out SARS-CoV-2 qualitative proficiency testing for domestic testing laboratories, and have raised issues such as insufficient overall testing capabilities and lack of personnel training. But there has been little discussion of quantitative capabilities and regulatory regimes.
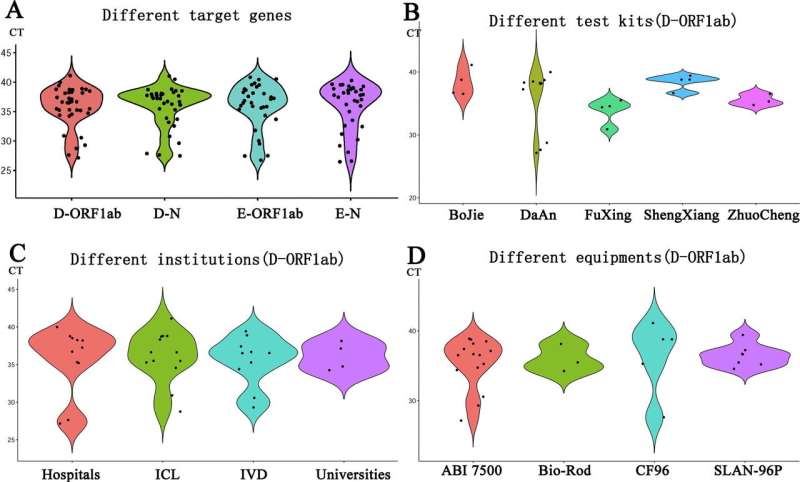
For quantitative capabilities, dPCR methods are more comparable and reliable, while qPCR results are easily affected by the use of standard materials and personnel operation. Quantitative competence is the basis for qualitative judgment. Participants from hospitals reported more satisfactory outcomes, while participants from businesses were less satisfied.
The systems such as "CE" certification based on "self-declaration" are temporarily not suitable for China, and national supervision and the development of various verification projects play an important role in improving nucleic acid detection capabilities.
Not all laboratories have satisfactory results with SARS-CoV-2 testing, especially those from ICL. It is necessary to further improve quality systems, including selection of qualified testing kits, personnel training, standardized operations, daily quality control, and regular external assessments. Improving the overall testing capacity of a laboratory can greatly avoid the occurrence of testing errors and provide help for epidemic prevention and control.
More information: Yongzhuo Zhang et al, Evaluation of factors contributing to variability of qualitative and quantitative proficiency testing for SARS-CoV-2 nucleic acid detection, Biosafety and Health (2022). DOI: 10.1016/j.bsheal.2022.08.004