Treatments compared for pediatric-onset multiple sclerosis
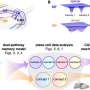
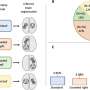
More patients with pediatric-onset multiple sclerosis (POMS) are free of new or newly enlarging (N or NE) T2 hyperintense lesions with dimethyl fumarate (DMF) versus interferon β-1a (IFNβ-1a) treatment, and the annualized relapse rate is lower with DMF, according to a study published online Sept. 28 in JAMA Network Open.
Patrick Vermersch, M.D., Ph.D., from the Centre Hospitalier Universitaire de Lille in France, and colleagues conducted an active-controlled, open-label randomized clinical trial involving patients with POMS aged 10 to <18 years. A total of 150 patients with POMS were randomly assigned to DMF or IFNβ-1a (78 and 72 individuals, respectively).
The researchers found that among the 103 trial completers, the proportion of patients with no N or NE T2 hyperintense lesions at week 96 was 16.1 and 4.9 percent for DMF and IFNβ-1a, respectively; in a sensitivity analysis among the intention-to-treat population, the proportions were 12.8 and 2.8 percent, respectively. The estimated proportion of patients who remained relapse-free was 66.2 and 52.3 percent for DMF and IFNβ-1a, respectively, at week 96. The adjusted annualized relapse rate was 0.24 and 0.53 for DMF and IFNβ-1a, respectively, at week 96; the rate ratio was 0.46 for DMF versus IFNβ-1a.
"The CONNECT randomized clinical trial found that DMF led to meaningful improvements in radiological and clinical outcomes in patients with POMS, with a positive benefit-risk balance," the authors write.
Several authors disclosed financial ties to biopharmaceutical companies, including Biogen, which manufactures dimethyl fumarate and funded the trial.
More information: Patrick Vermersch et al, Effect of Dimethyl Fumarate vs Interferon β-1a in Patients With Pediatric-Onset Multiple Sclerosis, JAMA Network Open (2022). DOI: 10.1001/jamanetworkopen.2022.30439
Omar Abdel-Mannan et al, Considering the Future of Pediatric Multiple Sclerosis Trials After the CONNECT Open-Label Randomized Trial, JAMA Network Open (2022). DOI: 10.1001/jamanetworkopen.2022.30451
Copyright © 2022 HealthDay. All rights reserved.