Genetically modified mice pave the way for customized medicine in a rare disease
An international research team led by Dr. Ana Guadaño at the Alberto Sols Biomedical Research Institute (IIBM, a combined CSIC-UAM center) and involving the Complutense University of Madrid (UCM), used CRISPR gene editing techniques to incorporate into mice a mutation of the MCT8 protein responsible for transporting thyroid hormones to the interior of the cell.
Patients with mutations in this protein suffer from Allan-Herndon-Dudley syndrome, a rare disease that takes the form of serious neurological alterations, in which each patient may reveal a different mutation of MCT8.
This study, published in Neurobiology of Disease, describes the first avatar model for the disease—in other words, the first animal model with the same genetic alteration as various patients.
"The development of avatar models faithfully reproducing the patients' alterations with this same mutation is the first step towards targeted therapy. It specifically lays the foundations to allow us to study the possible 'genetic repair' of this mutation in an animal model, and to evaluate whether this serves to avoid or revert the serious neurological alterations which exist in such patients," says Carmen Grijota, researcher at the Cellular Biology Department of the UCM and IIBM.
Same neurological and motor alterations in avatars and humans
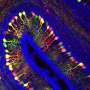
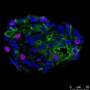
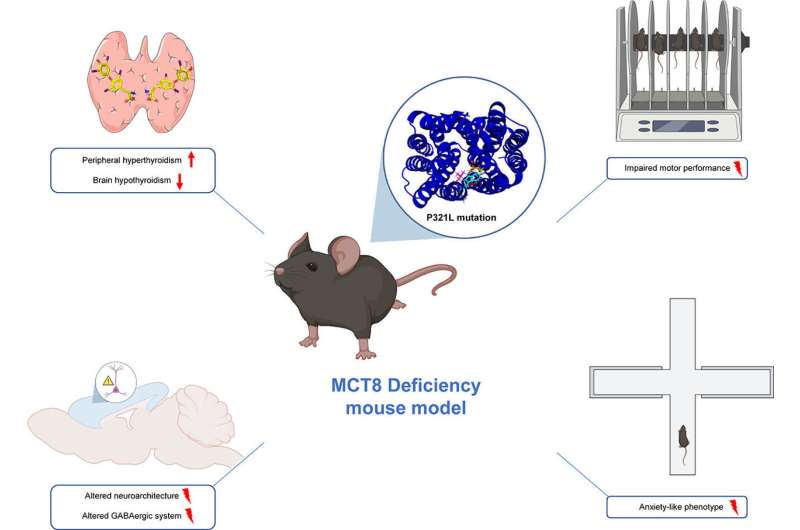
This study used mice carrying the "P321L" mutation. Tests were conducted to study the behavior, levels of anxiety and motor coordination capacity of the mice. The animals' brains were then extracted and specific stains applied to review and study different types of neuron.
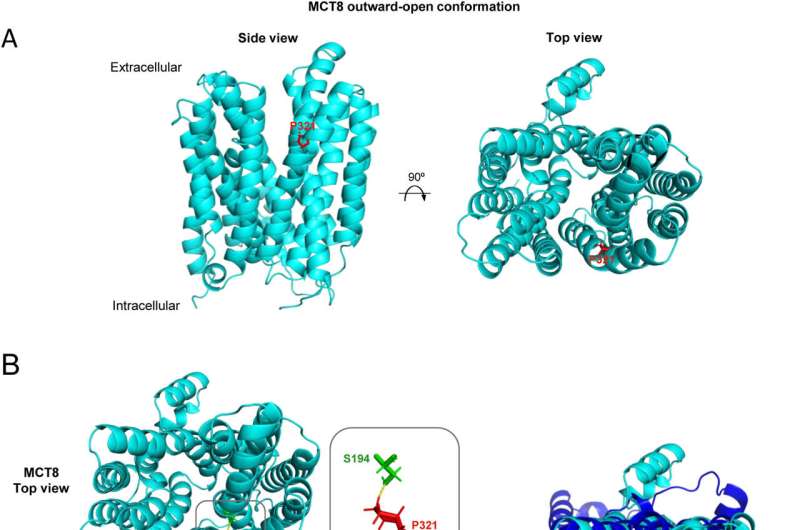
"Lastly, an in-depth computerized analysis was conducted to understand how the mutation could be affecting the structure of the MCT8 carrier, and hence its function of transporting thyroid hormones," says Víctor Valcárcel, IIBM researcher and co-author of the paper.
The alterations seen in the mice included cerebral hypothyroidism (lack of thyroid hormones in the brain), hyperthyroidism (excessive thyroid hormones in the other tissues), alterations in the distribution of the neurons in the cerebral cortex, and a reduction in GABAergic neurons. Alterations were likewise noted in motor coordination, as well as anxious behavior in the mutant mice. All these findings reflect the alterations characteristic of patients suffering from the disease.
According to the scientists, the next steps to be taken in their research would involve the administration of drugs that mimic the activity of thyroid hormones, but do not require MCT8 to enter the cells, because they use other different carriers. "The idea is to ascertain whether these drugs can reach the brain of the mutant mouse and improve all these alterations," explains IIBM researcher, Marina Guillén.
More information: Víctor Valcárcel-Hernández et al, A CRISPR/Cas9-engineered avatar mouse model of monocarboxylate transporter 8 deficiency displays distinct neurological alterations, Neurobiology of Disease (2022). DOI: 10.1016/j.nbd.2022.105896