January 23, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Characterizing soft biological tissue with new biomechanical testing methods in the lab
Soft biological tissues are important constituents that influence human physiology and disease since they impact cell behavior during tissue development, maintenance and repair. Most existing methods are limited by comprehensive characterization techniques to thereby impair fundamental processes underlying tissue architecture.
In a new report now published in Science Advances, Luca Rosalia and a research team in health sciences, physics and engineering, at Harvard and Cambridge developed an instrument for uniaxial tensile testing of a soft biological tissue in the lab, based on closed-loop interactions between an electromagnetic actuator and an optical strain sensor.
The team validated the instrument by using synthetic elastomers, then used the device to examine the mechanical properties of soft tissues such as murine esophageal tissue and its constituting layers, which include epithelial, connective and muscle tissues. The scientists improved the reliability of the instrument to facilitate an ideal platform for a wide-range of studies on the biomechanics of soft biological tissue.
Understanding the properties of soft biological tissue
Properties of soft tissues include stiffness, strength and viscoelasticity that are key to varying biological processes, including embryonic morphogenesis, postnatal development and physiological function. Such biological properties also play a role in initiating and progressing a variety of pathologies from cancer to wound healing and fibrosis, as well as cardiovascular diseases. However, the available mechanical data on biological tissues are sparse due to the limits of the existing methods of characterization. For instance, at present, the tensile properties of biological tissues can be primarily assessed using atomic force microscopy.
In this work, Rosalia and the team introduced the design and development of a tensile testing apparatus and validated its performance against conventional testing methods by using synthetic elastomers first with already known mechanical properties, followed by the biomechanical characterization of the murine esophagus and its constitutive layers to understand the performance of the newly developed device and determine the reliability of the method established in the lab.
Design considerations of the device
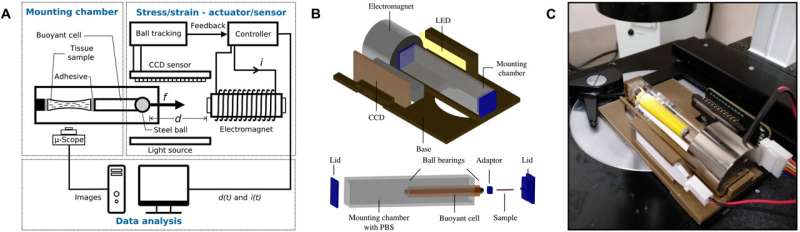
The researchers used the proposed instrument to test its biomechanical character at the millimeter scale. The parameters corresponded to the average size of human tissue samples routinely biopsied in the clinic and to embryonic mouse and adult tissue used in biomedicine. The team divided the architecture of the proposed instrument into three sections relative to their applications to include sample handling, the application of force and measurements of deformation.
The researchers designed the mechanical instrument to align its light source, electromagnet and mounting sample chamber in a reliable configuration. They integrated electrical and optical components of the device to simultaneously conduct tensile testing and live imaging of small biological tissue specimen. They also included a magnetic actuator within the device and an electromagnet to generate a variable magnetic field and an optical system.
Recreating a microphysiological environment in the lab
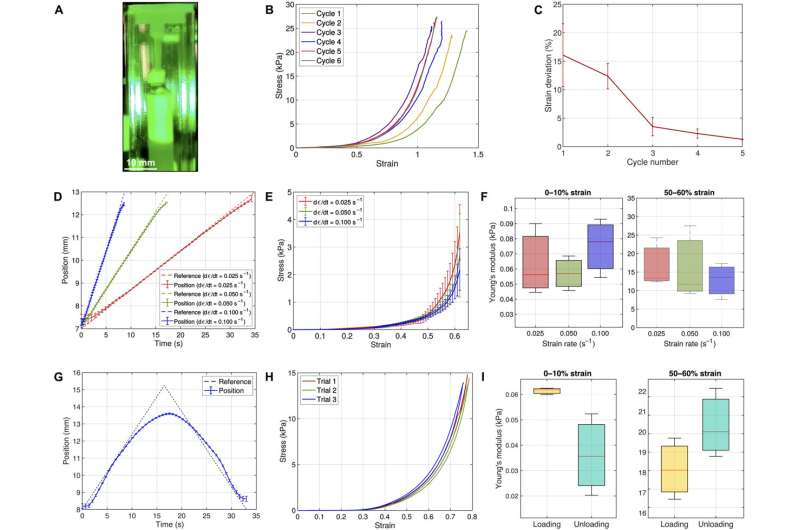
The team intended to test the biomechanics of soft biological tissue by maintaining them as closely as possible to their physiological environment. They achieved this by designing a transparent mounting chamber and immersed the test specimen tissue in a saline solution. They then designed a closed-loop feedback system to facilitate electromagnetic stability and mechanical properties arising from the sample, which included external sensors, vibrations or sensor noise.
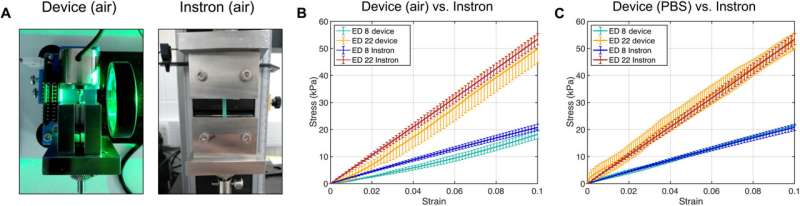
The scientists comparatively validated the device by assessing its performance with established methods, using polyvinyl siloxane on the instrument and on an Instron tensile tester. They next tested the mounting chamber to recreate the physiological environment of biological tissues accomplished with finite element modeling to further characterize the stress-strain response of the device.
Biomechanical characterization of the esophagus
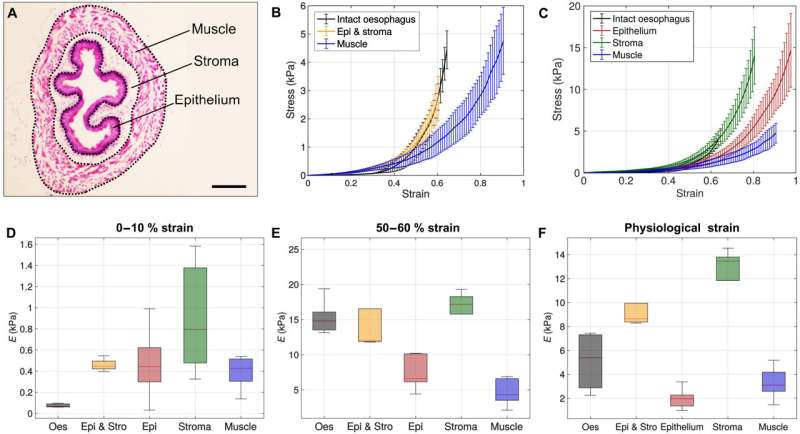
The team next identified the multiple tissue layers surrounding the esophagus, including the mucosa, submucosa and tunica muscularis. Using the device, they additionally conducted a first ever in-study uniaxial tensile testing technique to biomechanically characterize the entire esophageal tissue and its three main constituting layers. The mucosa contained a squamous stratified epithelium with differentiated suprabasal cells and self-renewing basal progenitor cells.
The bioengineers have yet to comprehensively characterize the mechanical behavior of the esophagus due to the absence of adequate testing methods within a research field largely limited to animal models. In this work, the team conducted the first uniaxial tensile testing method to biomechanically characterize the entire mouse esophageal tissue and its three constituting layers.
Outlook
In this way, Luca Rosalia and colleagues developed a high-fidelity device for uniaxial tensile testing of soft biological tissues. The device functioned as a closed loop system to generate a tensile force based on the interactions between an electromagnet and a ferromagnetic bead, while tracking the displacement of the sample under a variety of loading conditions. The scientists validated the device by characterizing the elastic properties of synthetic materials, followed by investigating the biomechanics of the mouse esophagus.
Future investigations can facilitate further measurements of the viscoelastic properties of soft biological tissues to ultimately support the informed decision-making process for diagnostic or prognostic outcomes during translational mechanobiology in clinical research.
More information: Luca Rosalia et al, A magnetically actuated, optically sensed tensile testing method for mechanical characterization of soft biological tissues, Science Advances (2023). DOI: 10.1126/sciadv.ade2522
Pei-Hsun Wu et al, A comparison of methods to assess cell mechanical properties, Nature Methods (2018). DOI: 10.1038/s41592-018-0015-1
© 2023 Science X Network