This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cross-sectional study finds systematic reviews are important for justifying new randomized clinical trials
Systematic reviews can help to justify a new randomized clinical trial (RCT), inform its design and interpret its results in the context of prior evidence. Therefore, the citation of systematic reviews in RCT reports have been promoted in the past two decades. However, it remains unclear whether such efforts have paid off.
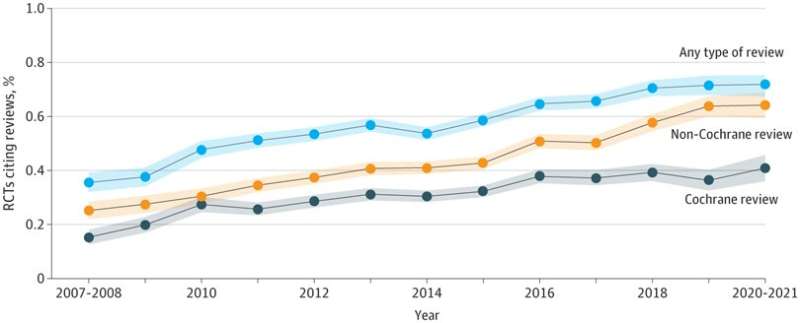
Recently, an international research team led by Drs. Tang Jinling, Jia Yuanxi from Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS), in collaboration with Dr. Karen A. Robinson from Johns Hopkins University, has revealed that the citation of systematic reviews in RCT reports has been improved. This study was published in JAMA Network Open on March 23.
The research team conducted a cross-sectional study to investigate 4,003 RCTs included in Cochrane reviews. Overall, 22,65 RCTs (56.6%) cited systematic reviews and 1,738 RCTs (43.4%) cited no systematic reviews. The percentage of RCTs citing systematic reviews increased from 35.5% before 2008 to 71.8% after 2020, with an annual increase of 3.0%.
"The overall percentage increases, but we cannot be too optimistic. On the one hand, almost 30% of RCTs published after 2020 still failed to cite systematic reviews; on the other hand, in some clinical fields such as ophthalmology, the percentage was as low as 26%," said Dr. Jia. "Some RCTs were at high risk of missing systematic reviews in their reports, such as those recruiting less than 100 participants, receiving non-industry funding, and conducted in low- or middle-income countries. We need to pay more attention to these RCTs in the future."
The current efforts may not be adequate. For example, although some journals have been requesting the citation of systematic reviews, the percentage of RCT reports citing systematic reviews in these journals did not differ from journals without such a request.
"These findings suggest that we must develop new or more aggressive methods to further improve the use of systematic reviews in RCT reports," said Dr. Jia.
More information: Yuanxi Jia et al, Trends of Randomized Clinical Trials Citing Prior Systematic Reviews, 2007-2021, JAMA Network Open (2023). DOI: 10.1001/jamanetworkopen.2023.4219