This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Research team develops new system for imaging and treating tumors
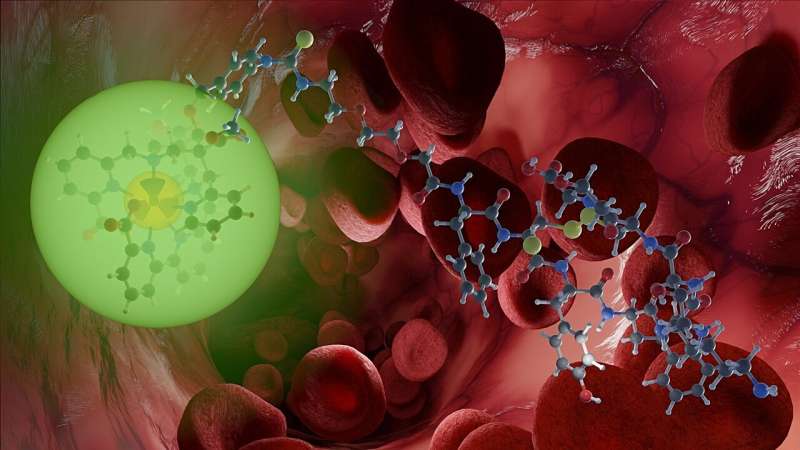
Thanks to the radiation they emit, radioactive compounds are suited both to imaging and treating cancers. By appropriately combining them in novel, so-called radionuclide theranostics, both applications can be dovetailed. A radiopharmacy team at Helmholtz-Zentrum Dresden-Rossendorf (HZDR) and Heidelberg University has now presented such a system in the Journal of the American Chemical Society that successfully solves one of the biggest problems to date: it works at physiologically relevant temperatures.
"Basically, we can think of it as functioning like a smart key that we use to control our automobiles. We use so-called radionuclides, i.e., unstable atomic nuclei, that spontaneously emit ionized radiation when they decay. We track down the tumor with a diagnostic radionuclide. The targeted internal irradiation close to the diseased tissue is then taken on by a different, therapeutic radionuclide," says Dr. Manja Kubeil of HZDR's Institute of Radiopharmaceutical Cancer Research, describing her theranostic approach.
Her team in the Department of Radionuclide Theragnostics develops exactly these types of substance to track and destroy tumors. Thus, the researchers employ matched pairs of radionuclides which, due to their decomposition characteristics, can be used both for imaging and for tumor therapy on the same target molecule.
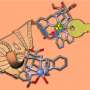
The relevant radionuclide is stably bound in what is known as a chelator and linked to a biomolecule by a kind of chemical bridge. "The word chelator comes from the Latin; its stem relates to being encircled by the claws of a crayfish. We prefer the image of a molecular cage that firmly encloses the radionuclide so that it can't spread in the body uncontrollably. The target-seeking biomolecule for its part must fit perfectly with the docking site on the cancer cells, just like a key in a lock. The radionuclide then accumulates on the tumor tissue and exclusively develops its destructive impact there—that's the idea," says Kubeil.
Stable bonds at practicable temperatures
Lutetium-177, for instance, is particularly suitable as a beta emitter for releasing electrons to treat various tumors as well as a source of gamma rays for imaging. Actinium-225, an alpha emitter that can be used for efficient treatment, is even more effective in destroying tumors and is also very tightly bound by the chelator. Neither radionuclide occurs naturally on Earth. Appropriate methods have to be used to produce them artificially.
Alpha emitters release particles composed of two protons and two neutrons. They are used in cancer therapy because their range in the tissue is very small, but they nonetheless attack and kill cancer cells very effectively thanks to their high energy. Their half-life of seven days in the case of Lutetium-177 and ten in the case of Actinium-225 is ideal for the purpose: it is long enough to enable effective treatment.
New chelator with advantages
So far, there has only been one complexing agent on the market that binds both radionuclides equally well: DOTA. The most frequently used chelator in nuclear medicine is known for its very stable metal complexes. But DOTA has one big disadvantage: only at what are very high temperatures for biochemical conditions, beyond 80°C, is it possible to bond theranostic radionuclides completely.
"If you are working with protein derivatives, these temperatures are way too high because even at 40°C denaturation kicks in: they are destroyed. Our new chelator system functions reliably at these lower temperatures," Kubeil says.
Moreover, under these milder conditions, it achieves faster radiolabeling than the known chelators. Another advantage is that the new system efficiently attaches to various bioconjugates. This means an increase in the choice of docking sites on diseased tissue. The newly developed chelator could thus form the basis for new modular and personalized pharmaceutical systems that could be geared towards different fields for imaging and therapy by simply exchanging partial chemical structures.
More information: Patrick Cieslik et al, Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225, Journal of the American Chemical Society (2022). DOI: 10.1021/jacs.2c08438