This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Study: Value of chemotherapy post immunotherapy in stage IV non-small cell lung cancer
A new research paper was published in Oncotarget, titled "Value of chemotherapy post immunotherapy in stage IV non-small cell lung cancer (NSCLC)."
Lung cancer is the number one cause of mortality among all types of cancer worldwide. Its treatment landscape has shifted from the classic chemotherapy alone to newer regimens based on the discovery of new immunotherapy and targeted therapy drugs. However, chemotherapy is still an option for treatment of advanced non-small cell lung cancer (NSCLC) after progression on immunotherapy alone or in combination with first-line chemotherapy.
This new retrospective study, by researchers Hazem I. Assi, Maroun Bou Zerdan, Mohammad Hodroj, Makram Khoury, Nour Sabiha Naji, Ghid Amhaz, Reine Abou Zeidane, and Fadi El Karak from the American University of Beirut Medical Center and Hotel Dieu de France University Hospital, was based on chart review of patients diagnosed with advanced NSCLC cases who received Docetaxel as second or third line after being treated by immunotherapy and/or chemotherapy in previous lines.
The data was collected from the medical records of physicians' clinics in three different hospital centers in Lebanon over the period of 5 years from July 2015 until December 2020. February 2021 was data analysis cut off time.
"The main aim [of this study] was to assess the role of Docetaxel post-chemoimmunotherapy for patients with diagnosed NSCLC," say the researchers.
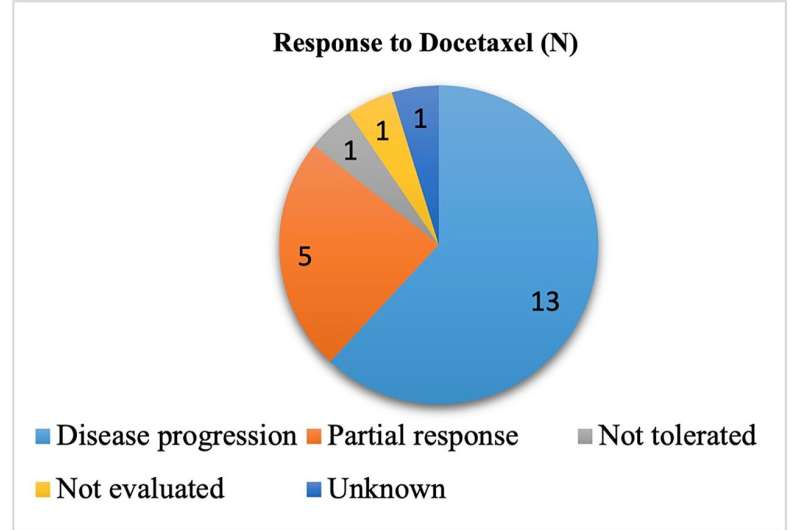
A total of 21 patients were included in this study. The majority of our patients were males (81%). As for histologic type, most patients had non-squamous lung cancer (67%) as compared to 33% who had squamous lung cancer. Overall, their study reported a 24% response rate to Docetaxel including stable disease and partial response and a median progression free survival (PFS) of 3 months. The mean time interval elapsed from diagnosis to the initiation of Docetaxel was 11.5 months.
"New therapeutic options should be validated for the treatment of NSCLC in the second and subsequent lines of therapy considering the poor prognosis of this disease. The chemotherapy in second and third line may keep an important role in the treatment after progression on newer agents, but it needs more evidence in prospective studies including a larger number of patients," say the researchers.
More information: Hazem I. Assi et al, Value of chemotherapy post immunotherapy in stage IV non-small cell lung cancer (NSCLC), Oncotarget (2023). DOI: 10.18632/oncotarget.28444