This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Modeling early heart failure could help researchers develop new treatments
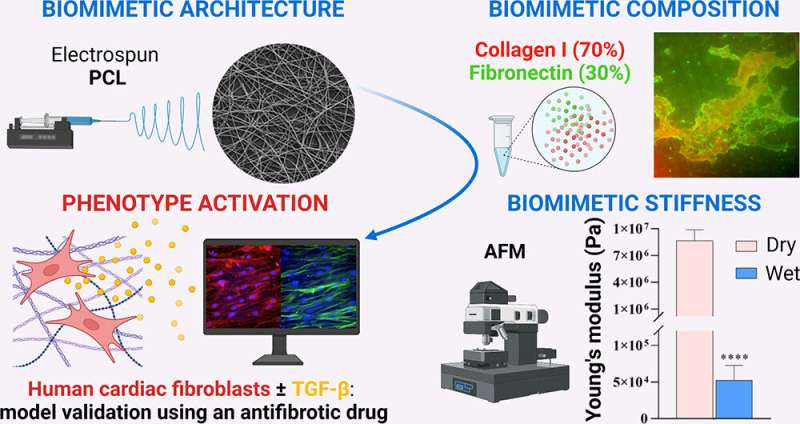
Heart failure is a common condition, though it currently lacks a cure. And treatments can only manage symptoms, often with side effects. But recent research published in ACS Biomaterials Science & Engineering could open up new pathways toward potential therapies by focusing instead on cardiac fibrosis—a condition that often precedes heart failure. Researchers have developed a more accurate model of this "scarred" cardiac tissue that could allow for quicker drug testing.
Millions of people are affected by heart failure, which occurs when the heart can't pump enough blood to support the body. Extreme cases can require surgeries or even whole-heart transplants, and these procedures can lead to complications.
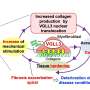
One underlying reason for heart failure is cardiac fibrosis—a condition in which stiff scar tissue forms around the heart and prevents it from beating properly. Though therapies for other aspects of heart failure are available, little attention has been paid to cardiac fibrosis as a potential target for new treatments.
As a result, there are also few testing platforms that recreate the unique aspects of the condition. Existing platforms have relied on animal cells, which lack some of the features of human cells, or involve hydrogels that don't match the architecture of scarred cardiac tissue. So, Gerardina Ruocco, Irene Carmagnola, Valeria Chiono and colleagues wanted to develop a more accurate in vitro model of human cardiac fibrotic tissue that could make future preclinical studies more effective and efficient.
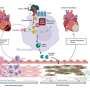
To do this, the researchers built a scaffold out of a biodegradable polymer using an electrospinning technique, building a network of randomly oriented nanofibers. The scaffold was dipped into a solution of collagen and fibronectin, which are important components of the cardiac environment that can contribute to fibrosis.
Human cardiac cells, which form stiff scar tissue when they differentiate into myofibroblasts, were allowed to grow on the scaffold. Previous tissue models required certain growth factors to induce this differentiation, but the new model did not—its rigid scaffolding caused this differentiation naturally.
To validate the model, the team tested its response to a drug with an antifibrotic effect. After administration, myofibroblasts did not form and the tissue remained flexible. The researchers say that this model could allow for more accurate testing of new fibrosis treatments, without the need for animals.
More information: Gerardina Ruocco et al, Biomimetic Electrospun Scaffold-Based In Vitro Model Resembling the Hallmarks of Human Myocardial Fibrotic Tissue, ACS Biomaterials Science & Engineering (2023). DOI: 10.1021/acsbiomaterials.3c00483