This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study offers insights into lymphatic system remodeling
Lymphangiogenesis refers to the formation and remodeling of lymphatic vessels (a network of thin tubes that carry lymph in the lymphatic system), and it supports the transport of molecules and immune cells around the body. Recently, a team led by researchers at Osaka University has uncovered important insights into lymphatic cell migration and lymphatic vessel remodeling.
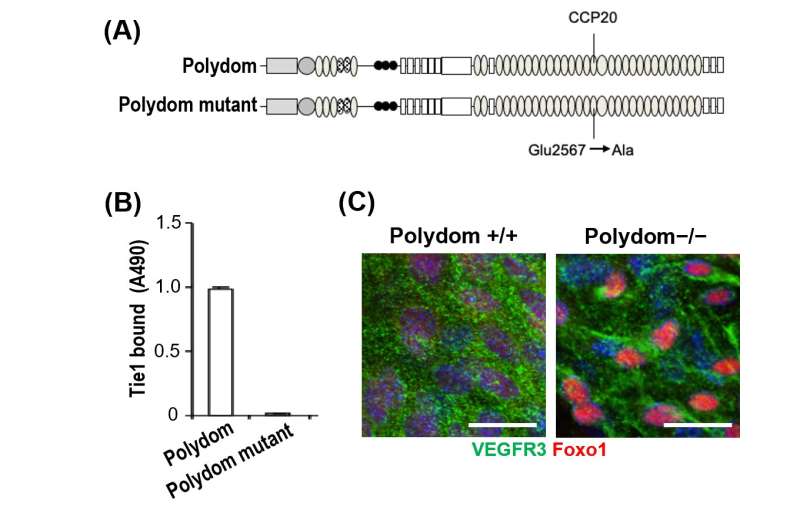
Polydom (or SVEP1) is a kind of protein that is essential for making new lymphatic vessels; this is known because mice that do not express this protein have problems with lymphatic development and do not survive after birth. However, not much is known about how this protein works in lymphangiogenesis, so the research team investigated this further. They identified that the binding of Polydom with another protein, Tie1, is important for lymphatic cell migration in lymphangiogenesis.
"We know that another protein, a receptor known as Tie1, is also important in the formation of lymphatic vessels, but it was unknown what proteins bind to Tie1 and, subsequently, which downstream signaling pathways they activate," explains lead author of the study Ryoko Sato-Nishiuchi. "Because mice lacking either Tie1 or Polydom have similar problems, we decided to investigate whether Tie1 and Polydom might interact in some way, to see how this relates to the process of lymphangiogenesis."
The research team first used a technique called solid-phase binding assay to show that Tie1 binds to Polydom. They then placed lymphatic endothelial cells (one of the key cell types that make up lymph vessels) into a special system to track cell migration, and found that both Tie1 and Polydom—but not other similar kinds of proteins—were essential for the migration of these cells.
"We were then able to identify some of the downstream pathways, such as the PI3K/Akt pathway and FoxO1 signaling, that are also important in the lymphatic endothelial cell migration induced by Tie1/Polydom," says senior author of the study Kiyotoshi Sekiguchi. "Our finding that the binding of Polydom and Tie1 facilitates lymphatic vessel remodeling by modulating the PI3K/Akt signaling pathway in lymphatic endothelial cells is important for understanding how lymphangiogenesis works."
A better understanding of lymphangiogenesis will aid in the development of treatments for diseases or injuries of the lymphatic system, such as lymphedema, which has no curative treatment. This disease can be genetic, and also commonly develops in cancer patients. Better treatments for lymphedema, as well as other lymph-related diseases, will improve the quality of life for many patients.
The article, "Polydom/SVEP1 binds to Tie1 and promotes migration of lymphatic endothelial cells," was published in Journal of Cell Biology.
More information: Ryoko Sato-Nishiuchi et al, Polydom/SVEP1 binds to Tie1 and promotes migration of lymphatic endothelial cells, Journal of Cell Biology (2023). DOI: 10.1083/jcb.202208047