This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Q&A: Dermatologist discusses FDA approval for alopecia areata treatment

Just a year after the U.S. Food and Drug Administration (FDA) approved the first treatment for severe alopecia areata, the federal agency has approved a second treatment for the disfiguring skin disease—both the result of pioneering research by the same Yale dermatologist.
On June 23, the FDA announced its approval for the use of ritlecitinib—a Janus kinase (JAK) inhibitor—to treat alopecia areata in both adolescents and adults. The medicine, taken orally, goes by the product name Litfulo.
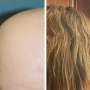
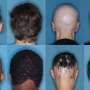
Alopecia areata is an autoimmune disease characterized by sudden, often disfiguring, loss of hair. It is the second most common cause of hair loss, affecting up to 7 million people in the United States.
Dr. Brett King, an associate professor of dermatology at Yale School of Medicine, worked with pharmaceutical company Pfizer to conduct a series of clinical trials with ritlecitinib. He worked with Eli Lilly and Company on clinical trials for the earlier medicine—baricitinib (which goes by the product name Olumiant), approved as a treatment for patients with severe alopecia areata in June 2022.
King's groundbreaking work with JAK inhibitors, which were originally designed to treat rheumatoid arthritis and myelofibrosis (a rare blood cancer), has shown significant potential to treat an array of intractable skin diseases, including eczema, erosive lichen planus, vitiligo, granuloma annulare, and sarcoidosis.
King spoke with Yale News about this latest FDA approval.
How does FDA approval for ritlecitinib change the treatment landscape for people with alopecia areata?
Brett King: Ritlecitinib [Litfulo] changes the treatment landscape for people with alopecia areata enormously. Last year, history was made when baricitinib [Olumiant] was FDA approved for the treatment of adults with severe alopecia areata. But alopecia areata affects people of all ages and, indeed, it commonly affects children of all ages. Ritlecitinib is approved in patients ages 12 years and older.
Childhood and adolescence are such vulnerable times, and children and adolescents have so much to do and learn and become during these years. It is challenging enough to be a kid, but when alopecia areata happens and suddenly one has big bald spots or is completely bald and missing eyebrows, the normal trajectory of that kid's life, and the family's life, too, can be derailed. Kids withdraw from sports and other social activities, and even from school. Extreme sadness and anxiety are common. It is awful. There is a way out of the darkness, however, and that is to regrow the hair that was lost, to restore the person as they had been prior to alopecia areata.
Normalcy is so important for everybody, but especially when we are developing. So it is easy to understand what a monumental breakthrough it is to have a medicine, ritlecitinib, approved for adolescents. Ritlecitinib restores normalcy and will make life better—literally will change life—for so many people.
When can patients in the U.S. expect ritlecitinib to be available for use?
King: Hopefully in the days or weeks ahead.
You have been at the center of two FDA approvals for major treatments of alopecia areata in two years. Has that sunk in yet—and how does that make you feel?
King: These new medicines for alopecia areata are historic, and I feel super fortunate to be a part of their development. Being a doctor is amazing because I get to share in the lives of others, hopefully making those lives better. It happens one person at a time, though. To have played a central role in the development of treatments for alopecia areata and other diseases—treatments that doctors around the world will give to thousands and thousands (or even millions) of people to make their lives better—is really incredible. We are all a part of something bigger than ourselves, and for me this experience highlights that as well as the possibility that we can change the world.
What are you working on next?
King: The next horizon is approval of these and other treatments for younger patients. Remember, alopecia areata is not uncommon in pre-adolescents. Also, JAK inhibitors do not work for everybody with alopecia areata, and so work needs to be done both to understand why that is and to develop treatments other than JAK inhibitors. The goal is for everybody to be able to have effective treatment. We have come so, so far but we still have a ways to go. It's exciting.