This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Designing surfaces to improve bone grafts
The field of bone implants has taken incredible strides thanks to technological innovations that allow for stronger grafts that are easier to install. Yet even with these advances, there are still risks involved in such procedures. Implants can be loosened following operations, for example, which can lead to costly surgical revisions that lengthen the recovery process for patients.
New research published in Nature Biomedical Engineering from an interdisciplinary team from Northwestern Engineering's Center for Advanced Regenerative Engineering (CARE) and Center for Physical Genomics and Engineering (CPGE) could reduce the likelihood of these painful, expensive complications.
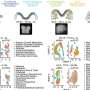
Working at the convergence of the physical sciences, biology, surgery, and engineering, the investigators introduced the concept of surface topography-induced chromatin engineering. In a collaboration with The University of Chicago's Russell R. Reid, MD, Ph.D., and Tong-Chuan He, MD, Ph.D., the team explained how and why to use surfaces to change patterns, validating the method in vivo.
The findings could lead to new design strategies for biomedical companies to improve the performance of medical devices for musculoskeletal surgery.
"We could potentially design implant surfaces that would promote significantly better integration with surrounding bone, for example tissue fixation devices and hip or knee implants," said Guillermo Ameer, ScD, the Daniel Hale Williams Professor of Biomedical Engineering at the McCormick School of Engineering and professor of Surgery at the Feinberg School of Medicine.
"This could also potentially be used to develop next generation bioresorbable scaffolds that better support new bone formation, by implementing these engineered topographies throughout the surface area of the implants."
Ameer and Vadim Backman, PhD, the Sachs Family Professor of Biomedical Engineering at the McCormick School of Engineering and of Biochemistry and Molecular Genetics and of Medicine at the Feinberg School of Medicine, were the paper's co-corresponding authors. Ameer and Backman are the directors of CARE and CPGE, respectively. Xinlong Wang, PhD, a postdoctoral fellow from CARE, and Vasundhara Agrawal, a PhD student from CPGE, were the co-first authors.
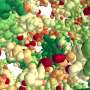
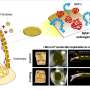
It's known from bench experiments that implant surfaces with engineered topographies can facilitate the differentiation of marrow-derived stem cells into bone-forming cells. The team demonstrated improved bone formation in vivo using implants with micropillars topography that induces cell nuclei deformation and facilitates the differentiation of marrow-derived stem cells into bone-forming cells.
The investigators did this in a critical-sized cranial defect model, where they showed that defects treated using implants with the micropillar surfaces had more bone formation than defects that received implants with a flat surface.
"Our research with the convergence of microfabrication, chromatin imaging, and bioimplants is the first application of chromatin engineering for regenerative medicine," Wang said. "The next step is to fabricate large-scale scaffolds that can be used for clinical translation."
"Although people have studied how surface chemistries and morphologies can affect cell shapes and function for decades, we are the first to demonstrate the direct role of deforming the nucleus of the cell and how that change leads to improved differentiation of mesenchymal stem cells—bone marrow derived—into the bone forming cells osteoblasts," Backman said.
How cells function depends not only on their genes but also on how these genes are expressed, and the latter is regulated by epigenetics. One critical aspect of epigenetic regulation is the 3D structure of the genome (chromatin). If genes can be compared to cells' hardware, epigenetics is software, and chromatin structure is the operating system.
The advent of CRISPR has ushered powerful tools for genetic engineering (designing the hardware). The next frontier is chromatin engineering or designing/reprogramming of the operating system.
CPGE was a natural fit for this study since it works to create new strategies for the treatment of disease and the reversible manipulation of physical systems through physical genomics.
"Our work demonstrated the possibility of leveraging mechanical cues to enable chromatin engineering for regenerative engineering. A unique aspect of our approach is that we focus not only on molecular but also physical factors that drive chromatin states and the resulting transcriptional landscape and cell phenotype," Backman said.
"Specifically, mechanical cues can be used to change the conformation of chromatin, change the patterns of gene expression, and foster faster differentiation of mesenchymal stem cells into osteoblasts, the cells critical for regeneration of the bone and cartilage tissues."
Ameer's work, meanwhile, specializes in regenerative engineering. He is on a mission to use engineering to enable the practice of regenerative medicine to improve the outcome of surgeries and benefit patients.
"Engineering the topography of implants to control cell responses in the body to promote regenerative processes (bone in our work) demonstrates a new way to design regenerative biomaterials and implants," Ameer said.
"Traditionally, research in our field focuses on including growth factors or other molecules that promote bone growth into the biomaterial or implant, but these approaches can have undesirable side effects and are difficult to bring to market. Our findings can be applied to musculoskeletal implants currently on the market, for example, the new tissue fixation devices made from citrate-based biomaterials pioneered by us, and now marketed by Stryker Corporation."
More information: Xinlong Wang et al, Chromatin reprogramming and bone regeneration in vitro and in vivo via the microtopography-induced constriction of cell nuclei, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01053-x