This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Uncovering why some cells become resistant to anti-cancer therapies
A research team led by Northwestern University and the University of Pennsylvania scientists has created a new synthetic biology approach, a "QR code for cancer cells," to follow tumor cells over time, finding there are meaningful differences in why a cancer cell dies or survives in response to anti-cancer therapies.
Remarkably, what fate cancer cells choose after months of therapy is "entirely predictable" based on seemingly small, yet important, differences that appear even before treatment begins. The researchers also discovered the reason is not genetics, contrary to beliefs held in the field.
The findings were published in the journal Nature.
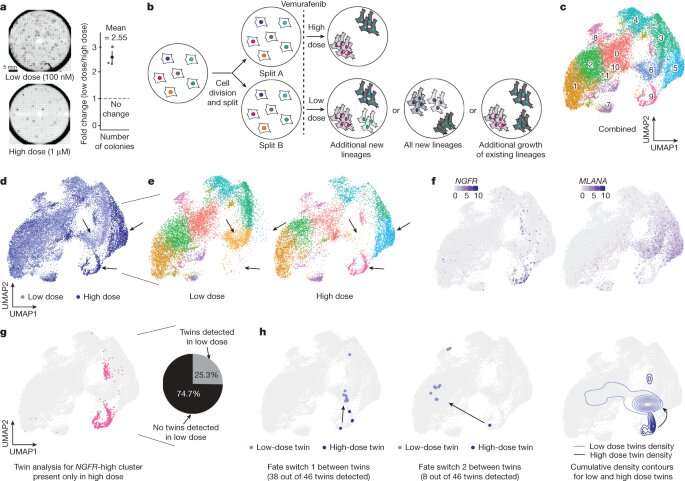
The study outlined the team's new technology platform that developed a "QR code" for each of the millions of cells for scientists to find and use later—much like tagging swans in a pond. The QR code directs researchers to a genome-wide molecular makeup of these cells and provides information about how they've reacted to cancer treatment.
"There are many ways cells become different from each other," said Yogesh Goyal, the paper's lead and co-senior author. "Our lab asks, how do individual cells make decisions? Understanding this in the context of cancer is all the more exciting because there's a clinically relevant dichotomy: A cell dies or becomes resistant when faced with therapies."
Goyal is an assistant professor of cell and developmental biology at Northwestern University Feinberg School of Medicine and has courtesy appointments with chemical and biological engineering and biomedical engineering at the McCormick School of Engineering. He is a member of Northwestern's Center for Synthetic Biology and the Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Goyal shares the senior author title with Arjun Raj, a geneticist at the University of Pennsylvania.
In the study, the lab and collaborators sought to apply synthetic biology tools to answer a key question in cancer research: What makes certain tumors come back a few months or years after therapy? In other words, could the lab understand what causes some rare cells to develop therapeutic resistance to a drug?
Some scientists in the field think genetic differences in the DNA of rare cells are what drive drug resistance. To investigate this hypothesis, the team put the before-and-after cloned cells through a whole genome sequencing pipeline to compare the populations and found no systematic underlying genetic mutations.
"We think this work stands to really change how we think about therapy resistance," said Arjun Raj, co-senior author and Professor of Bioengineering in the School of Engineering and Applied Science at the University of Pennsylvania. "Rather than drug resistant cells coming in just one flavor, we show that even in highly controlled conditions, different 'flavors' can emerge, raising the possibility that each of these flavors may need to be treated individually."
Using the interdisciplinary team—as well as his own training as an engineer with a strong mathematics background—Goyal helped develop the "QR code" framework, FateMap, that could identify each unique cell that seemed to develop resistance to drug therapy. "Fate" refers to whether a cell dies or survives (and if so, how), and the scientists "map" the cells across their lifespan, prior to and following anti-cancer therapy.
FateMap is the result of work from several research institutions, and it applies an amalgamation of concepts spanning several disciplines, including synthetic biology, genome engineering, bioinformatics, machine learning and thermodynamics.
"Some are different by chance—just as not all leaves on a tree look the same—but we wanted to determine if that matters," Goyal said. "The cell biology field has a hard time defining if differences have meaning."
By gathering data from before-and-after treatment, the scientists found that what cells do is completely determined prior to drug exposure. Finding differences in cells before adding a drug could therefore lead to the development of new therapies that target the driver of differences, rather than the result of it.
Goyal said their next set of questions lies in whether cell behavior can be predicted across multiple different cell therapies and cancer types. Do the same cells become resistant to treatment in other models or is a different set of cells impacted?
"This is just the beginning," Goyal said. "I expect the conceptual and technological advances from our work to be readily generalizable to disparate biological problems, from cancer to embryo development to regenerative medicine. Our work emphasizes the need to use approaches that seamlessly tread disciplinary boundaries to develop lasting treatments for cancer and beyond."
More information: Arjun Raj, Diverse clonal fates emerge upon drug treatment of homogeneous cancer cells, Nature (2023). DOI: 10.1038/s41586-023-06342-8. www.nature.com/articles/s41586-023-06342-8