This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
New approach to screening has potential to extend survival in glioblastoma brain cancer
A new form of screening may improve survival rates among people with a fast-growing type of brain tumor by helping identify those most likely to benefit from certain treatments.
Innovative preclinical research in mice models has shown that a molecular imaging technique can reveal the presence of a protein called PD-L1 in glioblastomas—the most common type of cancerous brain tumor in adults.
Alongside other measures, tests to detect high levels of PD-L1 could help direct treatment decisions, potentially leading to better patient outcomes.
Assessing PD-L1 expression levels
Currently, scientists assess PD-L1 expression levels by carrying out immunohistochemistry on samples of tissue taken from the patient during surgery, which is the first-line treatment for glioblastoma. However, this technique is subject to human error and is not standardized globally for these patients or this particular tumor. It can also be difficult to quantify the results.
Researchers at The Institute of Cancer Research have now shown that a non-invasive imaging technique called immuno-positron emission tomography (immuno-PET) could be a better approach. The research has been published in the journal Cancers.
Immunotherapy may improve the treatment landscape in glioblastoma
Glioblastoma starts as a growth of cells in the brain. It grows quickly and typically spreads within the brain, making it very difficult to treat effectively. No cure is yet available, and patients who initially respond to treatment tend to experience relapse. The average survival time is just 12–18 months, with only 5% of patients surviving more than five years.
In recent years, immunotherapy has shown potential as a treatment for glioblastoma. In particular, researchers have been testing drugs called immune checkpoint inhibitors, which prevent other proteins from dampening the body's immune response. The results to date have been mixed, suggesting that the treatment is only likely to be effective for a subset of patients.
Creating a novel radiotracer
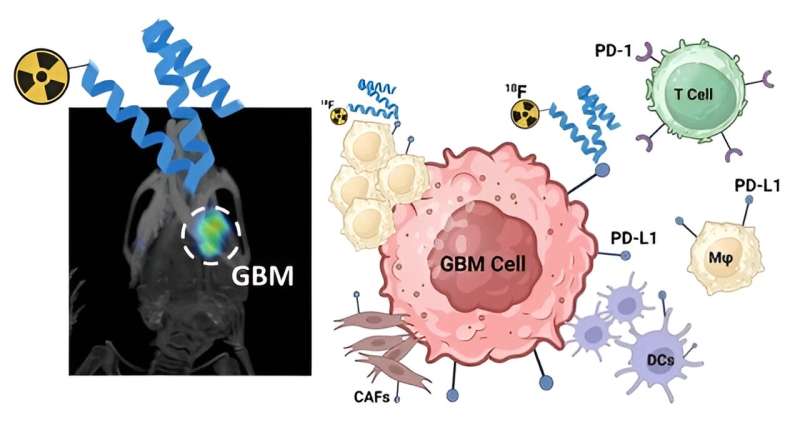
The ICR's team successfully used NOTA-maleinide to link ZPD-L1 affibody molecules to fluorine-18 and gallium-68 radionuclides. Affibodies are small proteins created to bind strongly to target proteins—in this case, PD-L1. This procedure created 18F-AIF-NOTA-ZPD-L1 and 68Ga-NOTA-ZPD-L1, which, with high specificity, recognize PD-L1 on tumor cells and in their microenvironment.
The team chose to use affibodies rather than antibodies because their much smaller size means that they clear the body far more quickly, minimizing the radiation dose for patients and preventing delays to surgery. Using affibodies also makes it possible to get high-quality images just one hour after injection. In comparison, when antibodies are used, the images are usually only retrieved after 48 hours.
Trialing the new approach
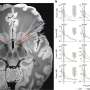
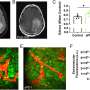
The researchers demonstrated that these radio-labeled affibodies could be used to assess the expression level of PD-L1 in tumors in mice. PET scans showed that although there was some uptake of the radiotracer in healthy tissue, the brain tumors were clearly visualized with high tumor-to-background contrast.
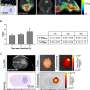
Then, the researchers looked into 36 samples from people with newly diagnosed glioblastoma. They noted PD-L1-positive membrane staining in 39% of the samples. A separate analysis of 161 human glioblastoma samples confirmed that tumors with a mesenchymal signature, which is linked to a better response to immune checkpoint inhibitors, had a significantly elevated expression level of PD-L1 compared with other glioblastoma subtypes. This supports the thinking that health care professionals could use immuno-PET to identify the patients most likely to benefit from immune checkpoint inhibitors.
The researchers hope that this work will lead to better outcomes for the 30%–49% of patients with the mesenchymal subtype of glioblastoma. They are now working on a clinical trial in Poland that builds on the foundations laid by this preclinical research and expect to present data from that trial in the near future.
Dr. Gabriela Kramer-Marek, Group Leader in Preclinical Molecular Imaging at the ICR, said, "It has been really exciting to see the journey from lab to clinic. We are currently running a clinical trial in people, which was only possible because of this promising preclinical work. The trial was the first ever to use immuno-PET to evaluate PD-L1 in people with primary glioblastoma, and we hope to see images that clearly show the presence of PD-L1 in these brain tumors.
"The treatment for glioblastoma has not changed for decades. Although we still do not have a cure, I believe that this new screening approach could definitely change patient outcomes."
More information: Gitanjali Sharma et al, Immuno-PET Imaging of Tumour PD-L1 Expression in Glioblastoma, Cancers (2023). DOI: 10.3390/cancers15123131