This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers find COVID-19 causes mitochondrial dysfunction in heart and other organs
Since the beginning of the COVID-19 pandemic caused by the SARS-CoV-2 virus, researchers have been trying to determine why this virus creates such negative long-term effects compared with most coronaviruses.
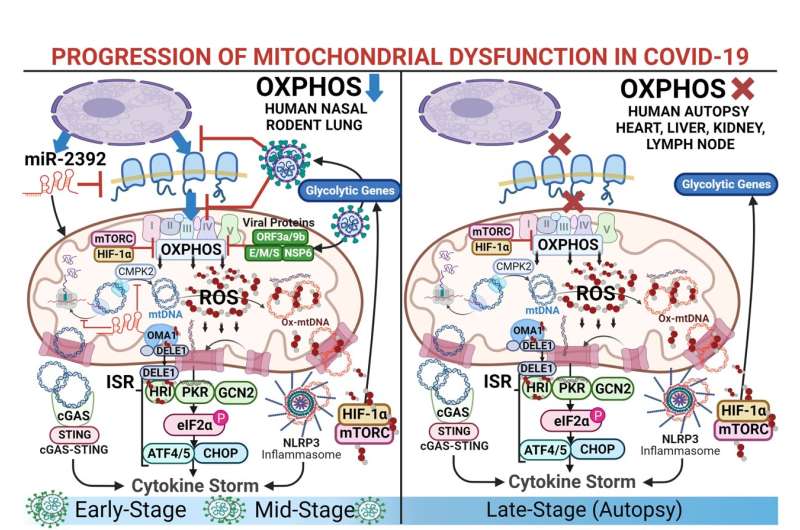
Now, a multi-institutional consortium of researchers led by a team at Children's Hospital of Philadelphia (CHOP) and the COVID-19 International Research Team (COV-IRT) has found that the genes of the mitochondria, the energy producers of our cells, can be negatively impacted by the virus, leading to dysfunction in multiple organs beyond the lungs. These findings, published by the journal Science Translational Medicine, suggest new approaches for treating COVID-19.
Mitochondria are found in every cell in our bodies. The genes responsible for generating mitochondria are dispersed across both the nuclear DNA located in the nucleus of our cells and the mitochondrial DNA (mtDNA) located within each mitochondrion. Prior studies have shown that SARS-CoV-2 proteins can bind to mitochondrial proteins in host cells, potentially leading to mitochondrial dysfunction.
To understand how SARS-CoV-2 impacts mitochondria, researchers from the Center for Mitochondrial and Epigenomic Medicine (CMEM) at CHOP along with their COV-IRT colleagues wanted to analyze mitochondrial gene expression to detect differences caused by the virus. To do this, they analyzed a combination of nasopharyngeal and autopsy tissues from affected patients and animal models.
"The tissue samples from human patients allowed us to look at how mitochondrial gene expression was affected at the onset and end of disease progression, while animal models allowed us to fill in the blanks and look at the progression of gene expression differences over time," said the study's first author Joseph Guarnieri, Ph.D., a postdoctoral research fellow with the CMEM at CHOP.
The study found that in autopsy tissue, mitochondrial gene expression had recovered in the lungs, but mitochondrial function remained suppressed in the heart as well as the kidneys and liver. When studying animal models and measuring the time when the viral load was at its peak in the lungs, mitochondrial gene expression was suppressed in the cerebellum even though no SARS-CoV-2 was observed in the brain. Additional animal models revealed that during the mid-phase of SARS-CoV-2 infection, mitochondrial function in the lungs was beginning to recover.
Taken together, these results reveal that host cells respond to initial infection in a way that involves the lungs, but over time, mitochondrial function in the lungs is restored, while in other organs, particularly the heart, mitochondrial function remains impaired.
"This study provides us with strong evidence that we need to stop looking at COVID-19 as strictly an upper respiratory disease and start viewing it as a systemic disorder that impacts multiple organs," said co-senior author Douglas C. Wallace, Ph.D., director of the CMEM at CHOP. "The continued dysfunction we observed in organs other than the lungs suggests that mitochondrial dysfunction could be causing long-term damage to the internal organs of these patients."
While future studies using this data will study how systemic immune and inflammatory responses may be responsible for more severe disease in some patients, the research team did find a potential therapeutic target in microRNA 2392 (miR-2392), which was shown to regulate mitochondrial function in human tissue samples used in this study.
"This microRNA was upregulated in the blood of patients infected by SARS-CoV-2, which is not something we normally would expect to see," said co-senior author Afshin Beheshti, Ph.D., a biostatistician, a visiting researcher at The Broad Institute, and founder and President of COV-IRT. "Neutralizing this microRNA might be able to impede the replication of the virus, providing an additional therapeutic option for patients who are at risk for more serious complications related to the disease."
Next, Dr. Wallace and CMEM will conduct research into how mtDNA variation among world populations might affect mitochondrial function and thus individual sensitivity to SARS-CoV-2. According to Wallace, the demonstration that SARS-CoV-2 markedly affects mitochondrial function supports the hypothesis that individual differences in mitochondrial function could be a factor in individual severity of COVID-19.
More information: Joseph Guarnieri et al, Core mitochondrial genes are downregulated during SARS-CoV-2 infection of mammalian hosts, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.abq1533. www.science.org/doi/10.1126/scitranslmed.abq1533