This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Expanding the impact of CAR T cell therapy: An immunotherapy strategy against all blood cancers
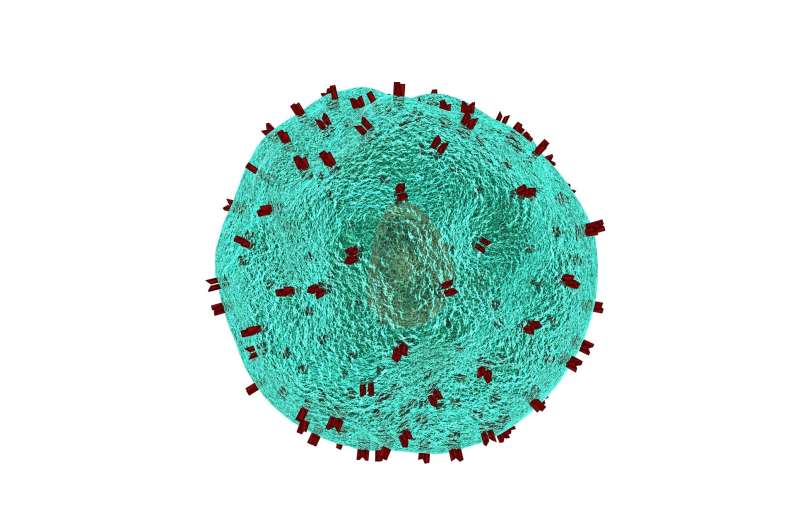
A broad new strategy could hold hope for treating virtually all blood cancers with CAR T cell therapy, which is currently approved for five subtypes of blood cancer. Scientists in the Perelman School of Medicine at the University of Pennsylvania have demonstrated the potential efficacy of this approach in preclinical tests.
In the study, published today in Science Translational Medicine, the researchers used engineered CAR T cells to target CD45—a surface marker found on nearly all blood cells, including nearly all blood cancer cells. Because CD45 is found on healthy blood cells too, the research team used CRISPR base-editing to develop a method called "epitope editing" to overcome the challenges of an anti-CD45 strategy, which would otherwise result in low blood counts, with potentially life-threating side effects.
The early results represent a proof-of-concept for epitope editing, which involves changing a small piece of the target CD45 molecule just enough so that the CAR T cells don't recognize it, but it can still function normally within the blood immune system.
"Up to this point, we haven't had the tools to create a targeted cell therapy approach that could work across all different forms of blood and bone marrow cancers," said senior corresponding author Saar Gill, MD, Ph.D., an associate professor of Hematology-Oncology. "We're excited to create a new solution that could solve a major issue in immunotherapy, which is the inability to target surface markers that are found on both cancer cells and healthy cells."
Each of the currently available cell-based immunotherapies for blood cancer is designed to work against a narrow range of malignancies based on their target antigens. For example, the first CAR T cell therapy, developed at Penn by Carl June, MD, the Richard W. Vague Professor in Immunotherapy, targets the CD19 protein marker on B cells, to treat B-cell lymphomas and leukemias.
Four of the six CAR T cell therapies currently approved to treat blood cancers target CD19. The other two target the BCMA protein marker to treat multiple myeloma. While CAR T cell therapy has been remarkably successful, researchers at Penn and across the world are working to make it even more effective for more patients.
"One drawback of the current approach to CAR T cell therapy is that each therapy must be developed individually based on the targets for that cancer type," said June, co-senior author of the study, who also directs the Center for Cellular Immunotherapies at Penn. "This study lays the groundwork for a more universal approach that could potentially expand CAR T cell therapy to all blood cancers."
Because CD45 is found on nearly all blood cells—and is usually highly expressed on blood cancer cells—a treatment that wipes out all CD45-bearing cells would leave patients without any blood cells, including red blood cells, platelets, plasma, and even the marrow-based stem cells that generate new blood cells. Furthermore, since T cells are blood cells and normally express CD45, CAR T cells targeting CD45 effectively would kill each other before they could be infused into patients.
The team built on previous work to overcome this challenge, using CRISPR base-editing to develop a new strategy called epitope editing. This involves the genetic modification of both the CAR T cells and blood stem cells to alter a small piece of the CD45 structure or "epitope" where the CAR T cells bind to the CD45 molecule. The altered version of CD45 still works but differs enough from normal CD45 that the anti-CD45 CAR T cells do not recognize and attack it.
"It's essentially a blood stem cell transplant paired with CAR T cell therapy," said lead author Nils Wellhausen, a graduate student in Pharmacology and a member of Gill and June's labs. "The idea is that when the engineered cells are infused, the CAR T cells kill the cancer cells that bear normal CD45, but don't kill each other or the newly engineered blood stem cells. This allows the engineered blood stem cells to begin making new blood cells."
Because the strategy results in replacing the stem cells that create new blood cells, it also has potential use as a milder form of chemotherapy conditioning, which is given to patients before a bone marrow transplant to suppress the immune system.
The researchers tested the strategy in an extensive set of experiments in cell culture and mice models. They showed that the new approach not only keeps anti-CD45 CAR T cells from attacking each other or stem cells, but also enables swift destruction of blood cell cancers. In one test, the anti-CD45 CAR T cells eliminated leukemia cells within three weeks of infusion and were still present and capable of killing leukemia cells more than two months later.
Further toxicology studies and additional modeling studies are currently underway in preparation for an investigational new drug application before it can move into Phase I clinical trials.
More information: Nils Wellhausen et al, Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adi1145. www.science.org/doi/10.1126/scitranslmed.adi1145