This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
An important step toward next-generation probiotics
One of the beneficial gut bacteria residing in the human gut, which normally cannot survive in an environment with oxygen, can now be made oxygen-tolerant. This is a key finding in the development of future probiotic treatment that is now being explored to improve glucose control in individuals with prediabetes.
Our intestines are home to trillions of bacteria, the gut microbiota, which are important for functions such as digesting food and educating and activating the immune system. During the past decade it has been clarified that changes in the bacterial composition can be linked to various diseases.
Significant expectations have been attributed to the next generation probiotics, or live bacteria products, which can replace the missing bacteria in individuals with increased risk of developing diseases. However, a significant problem has been to overcome the bacteria's oxygen sensitivity, since the vast majority are strictly anaerobic. With bacteria dying just seconds after being exposed to oxygen in the air, it has been hard to develop live bacterial cultures of extremely oxygen sensitive bacteria.
A naturally occurring symbiosis
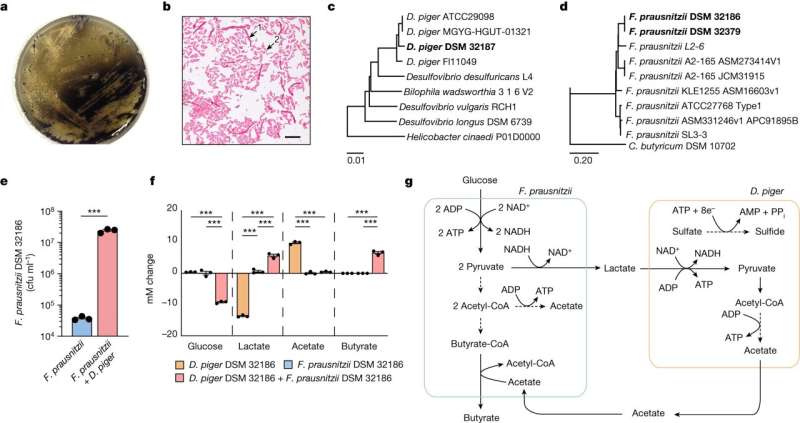
In a paper published in the journal Nature, researchers from the University of Gothenburg and the probiotic company BioGaia AB report how they have overcome the oxygen sensitivity of an anti-inflammatory gut bacterium, Faecalibacterium prausnitzii, which is significantly reduced in conditions including type 2 diabetes and cardiovascular disease.
In their study, Faecalibacterium prausnitzii was co-isolated with another bacterium, Desulfovibrio piger, which has beneficial effects on Faecalibacterium prausnitzii growth and function. By subsequently "training" the oxygen-sensitive bacteria in a favorable electrochemical environment, the researchers were able to isolate more oxygen-tolerant Faecalibacterium prausnitzii.
The team's leader, Professor Fredrik Bäckhed, explains, "By combining a naturally occurring symbiosis with 'training' of the bacteria, we have established a new strategy for producing otherwise oxygen-sensitive bacteria as live biotherapeutic products, which could prevent diseases when these bacteria are reduced in number."
Studying the impact on sugar control
The first author of the study is Muhammad Tanweer Khan, a researcher in Professor Bäckhed's team who previously discovered this symbiosis. He also represents the probiotic company BioGaia AB, which helped to develop the bacteria for the proposed dietary supplement.
"When these bacteria were grown together, both the biomass—in other words, the number of bacteria—and the production of butyrate, which has an anti-inflammatory effect, increased," he says. "This will enable us to increase the production yield, and to potentially increase butyrate production in the future."
The study shows that the combination of bacteria is safe for human consumption. The next step will be a study to investigate how supplementation with these bacteria may affect sugar control in individuals with prediabetes.
"We have high hopes," concludes Professor Bäckhed. "Further studies will show if we are correct in our hypothesis that gut bacteria have the potential to improve our health."
More information: Fredrik Bäckhed, Synergy and oxygen adaptation for development of next-generation probiotics, Nature (2023). DOI: 10.1038/s41586-023-06378-w